Part 10: Stereochemistry in Pharmaceutical Sciences – Current Trends and Future Directions
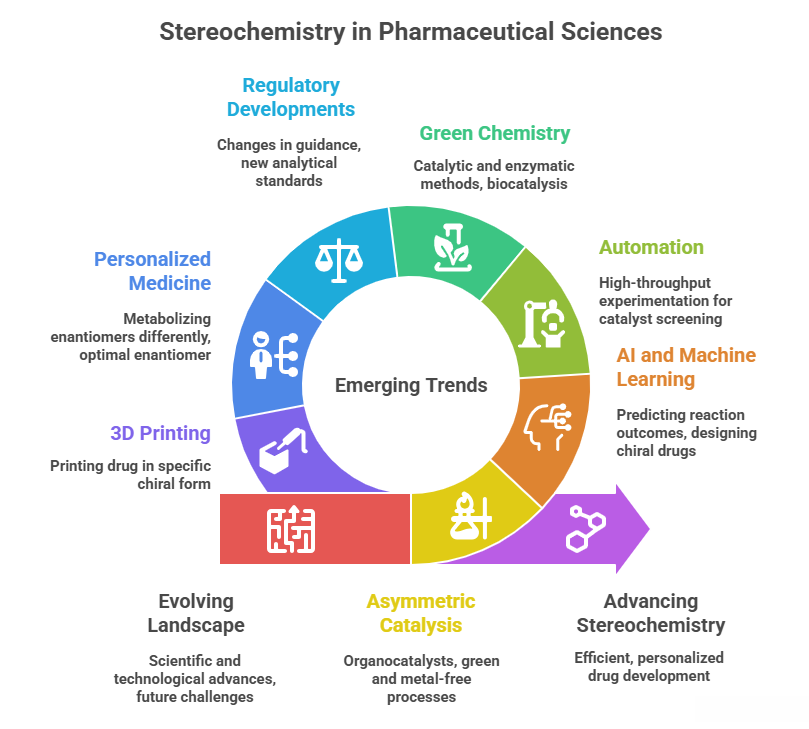
“Beyond today – emerging trends shaping the future of stereochemistry in pharma” Introduction The landscape of stereochemistry in pharmaceutical sciences continues to evolve with scientific and technological advances. This final part looks at emerging trends and future challenges. These include: – New developments in asymmetric catalysis, such as organocatalysts (MacMillan/List catalysts) that have expanded the toolkit for chiral synthesis, and the 2021 Nobel recognition of this field – implying more green and metal-free asymmetric processes …