Stereochemistry, in particular chirality, is known to us as early as 1809, when Malus, Arago and Biot discovered plane polarized light and its characteristics. It is observed that during 1950s to the 1970s, the “Golden Age” of drug discovery & development, stereochemistry was largely ignored resulting in approximately 57% of pharmaceuticals being marketed as racemates by the 1980s. Before going further let us examine the stereospecific awareness level that existed during 1980s’.
Stereospecific Awareness level
To understand the stereospecific awareness level Professor E.J.Arëins, conducted a study on books on subjects that is expected to have some discussion on stereochemical aspects. He examined authoritative books on pharmacokinetics, pharmacology, and toxicology from a stereochemical perspective and checked on three parameters namely:
(I) stereoselectivity & related terms in index
(II) discussions on implication of stereoselectivity
(III) avoidance of stereochemically biased data in the text
Then it is assigned the symbol ‘+’ if there is a discussion on the above points'(awareness level -good) and the symbol ‘-‘ if there is no mention (stereospecific awareness level – poor). Representative data are shown in the tables below.
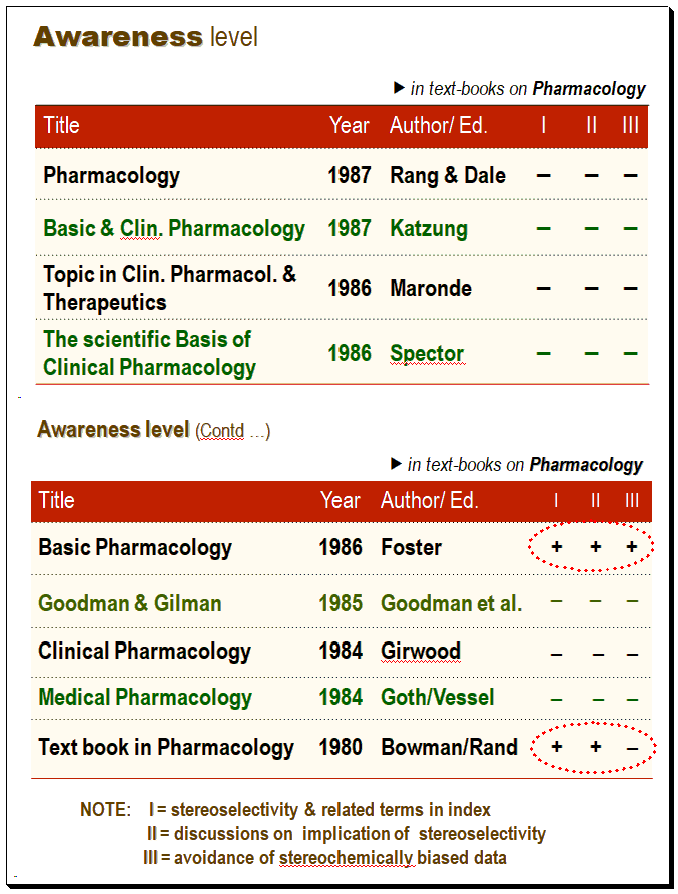
In the Pharmacology category it is found that only one book by Foster,1986, talks about all the thee parameters and assigned a (+) on all the three parameters. Another book by Bowman and Rand, 1980 talks about two of the the parameters. None of the rest books have any discussion on the stereochemical aspects reflecting a poor stereospecific awareness level at that point of time.
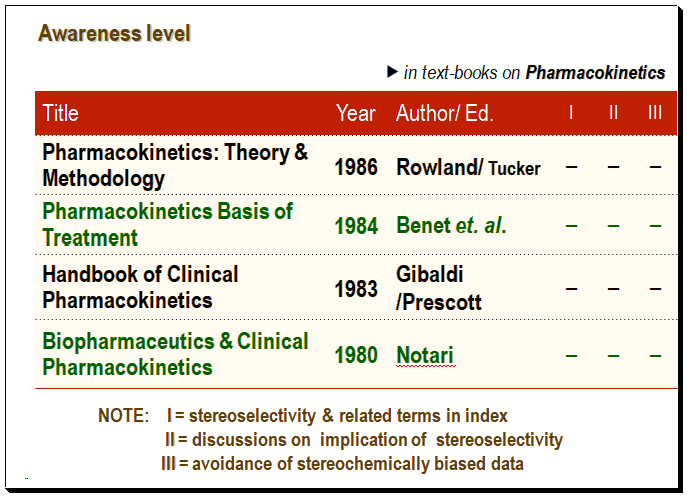
On the other hand the study on authoritative books on Pharmacokinetics it is found that none of them mention about the stereochemical parameters examined indicated by the (-) sign. Over all the literature search points to the fact that the stereospecific awareness level at that point in time was poor.
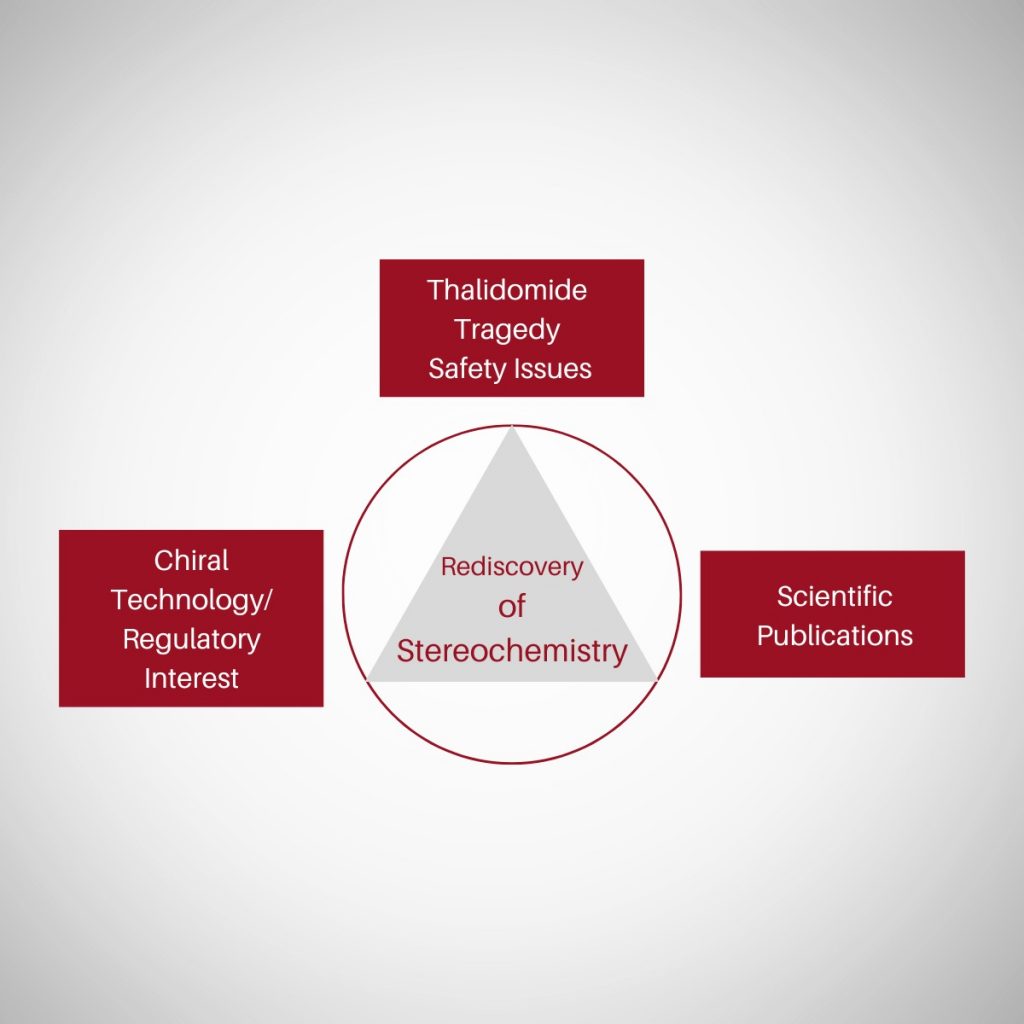
Rediscovery
Then, during the period late 1980s to 1990, there happened a Rediscovery of Stereochemistry. This could be attributed to four key events/stimulants namely (i) thalidomide tragedy (ii) landmark publications (iii) regulatory concerns and (iv) explosion of chiral technology.
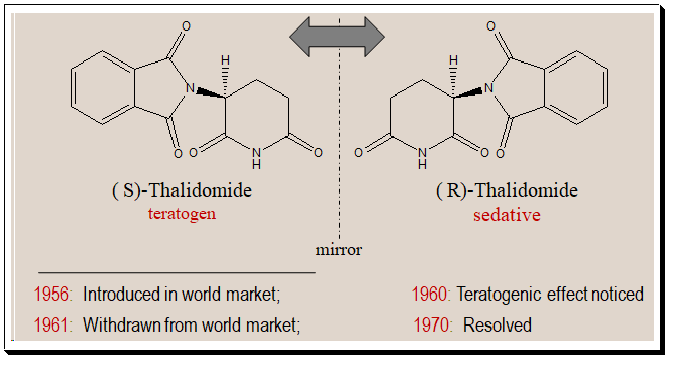
Thalidomide tragedy
Thalidomide tragedy led to profound and sustainable change in the ways we look at chiral molecules. Today enantiomers are treated as two different chemical entities at least with respect to chiral drugs, like a polypharmacy. Read more of thalidomide tragedy @ <https://chiralpedia.com/blog/thalidomide/>.
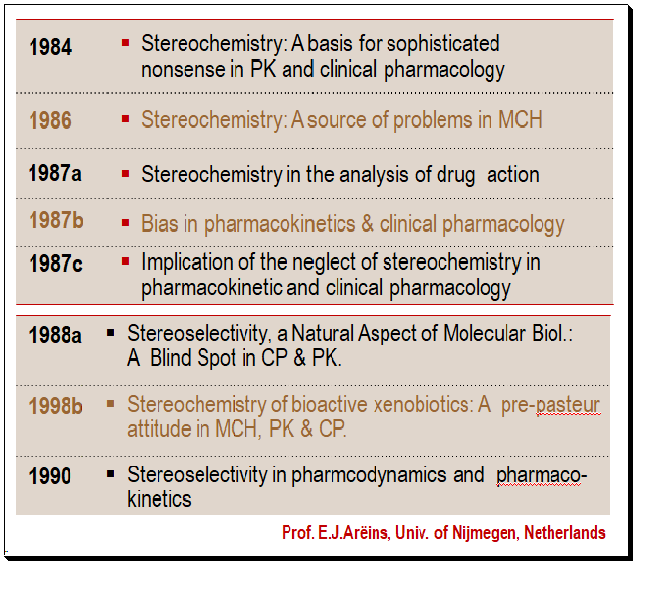
Breakthrough papers
Another important factor/event that possibly led to the rediscovery of stereochemistry was a series of articles published by Professor E.J.Arëins, Univ. of Nijmegen, Netherlands, criticized the practice of conducting PK and PD studies on racemic therapeutics and ignoring the separate contributions of the individual enantiomers. These papers have served to crystallize some of the important issues surrounding racemic drugs and stimulated much discussion in industry, government and academia. Some select scientific articles are given below.
Regulatory environment
The regulatory guidelines across the globe strongly encourage the development of single isomers and discourage stereoisomeric (e.g., racemic) mixtures. Approval could not be granted for a drug containing more than one isomer unless the PK and PD properties of each could be described and, more importantly, justified. USFDA, EU, DLA, Japan demand for enantiospecific data on PK, PD & TC profile of chiral drugs to asses the safety and efficacy. [ Chirality, 1992;4(5):338-40. DOI: 10.1002/chir.530040513
Chiral technology
Along with the change in regulatory control on stereoisomeric drugs there was an explosion of chiral technology in particular chiral analytical tools viz. Chiral separation and analysis techniques both on analytical and preparative scale. This enabled the separation and analysis of wide range of chiral molecules resulting in enantiospecific pharmacokinetic, pharmacodynamic investigations. New areas like steropharamcology also emerged. Parallelly, the filed of asymmetric synthesis also grew tremendously. In fact Nobel prize in chemistry was awarded in 2001 for the work on chirally catalyzed hydrogenation and oxidation reactions that has wide range of applications. and After two decades in 2021 for the development of asymmetric organocatalysis. Read more @ [https://www.nobelprize.org/prizes/chemistry/2001/summary/ and https://www.nobelprize.org/prizes/chemistry/2021/summary/]
Today, chirality become a vital tool in drug research and development.
References
Andrew J. Hutt, The development of single-isomer molecule: Why and how, Academic supplement, MedWorks Media, 14-22, 2022. https://medworksmedia.com/wp-content/uploads/2017/10/3-MF_Suppl_Hutt.pdf
E J Ariëns et al., Stereoselectivity of bioactive xenobiotics. A pre-Pasteur attitude in medicinal chemistry, pharmacokinetics and clinical pharmacology, Biochem Pharmacol, 1988 Jan 1;37(1):9-18. DOI:10.1016/0006-2952(88)90749-6
E. J. Ariens, E. W. Wuis, Clin. Pharmacol. Ther. 1987, 42, 361–363.
Ariëns,E.J. et. al., Biochem. Pharmacol.,1988, 37, 9.
Ariëns,E.J., Stereochemistry: A basis for sophisticated nonsense in PK and clinical pharmacology, Eur. J. Clin. Pharmacol.,1984, 26, 663.
Ariëns,E.J., Implication of the neglect of stereochemistry in pharmacokinetic and clinical pharmacology, Drug Intell. Clin. Pharm.,1987, 21, 827.
https://chiralpedia.com/blog/thalidomide
FDA’s policy statement for the development of new stereoisomeric drugs, Chirality,1992;4(5):338-40. DOI: 10.1002/chir.530040513.
https://www.nobelprize.org/prizes/chemistry/2001/summary
https://www.nobelprize.org/prizes/chemistry/2021/summary/
.