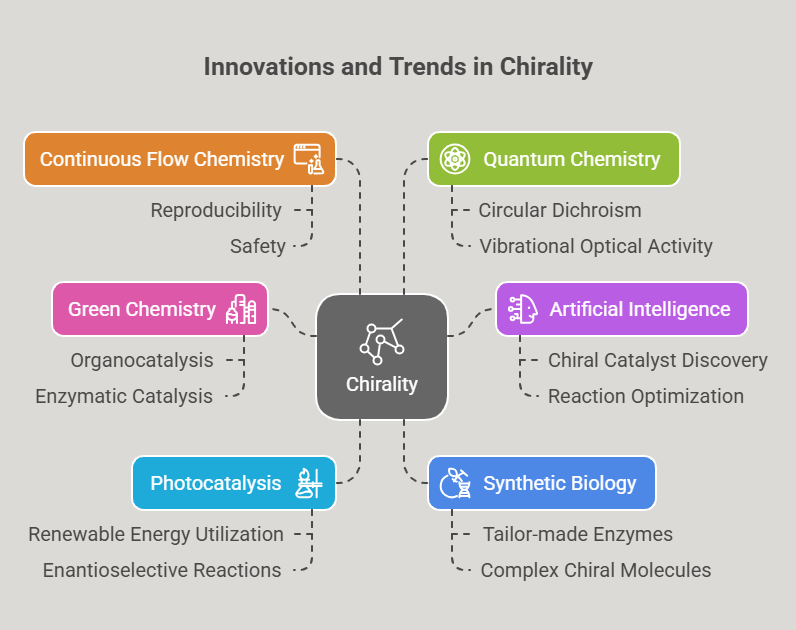
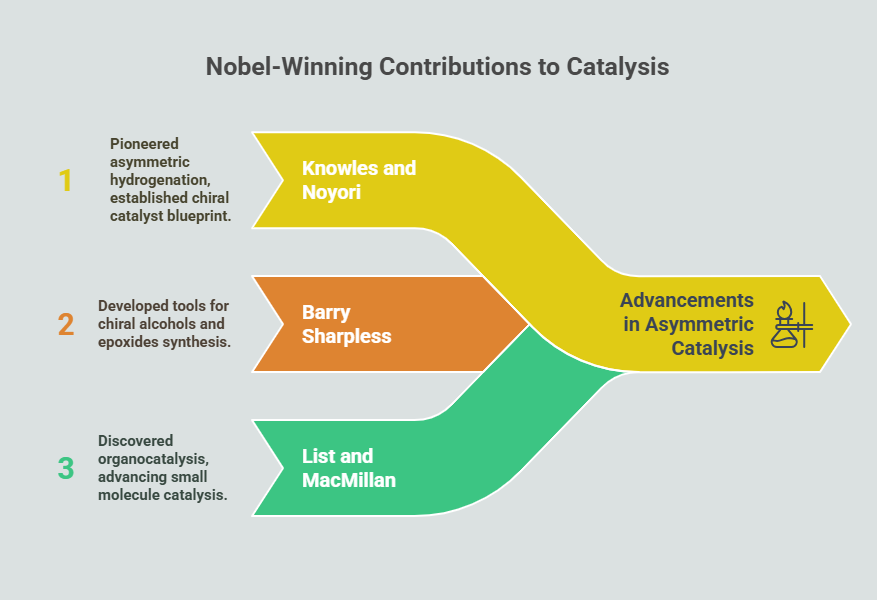
Episode 5: Choosing the Right Twin: Regulatory Expectations for Single Enantiomers
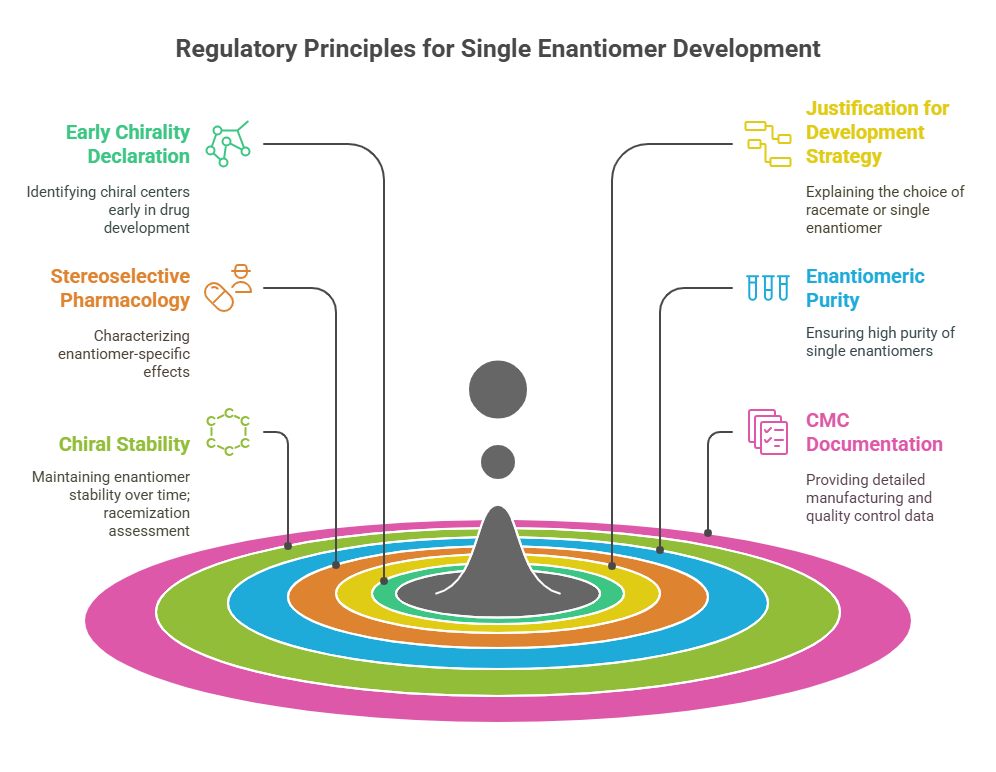
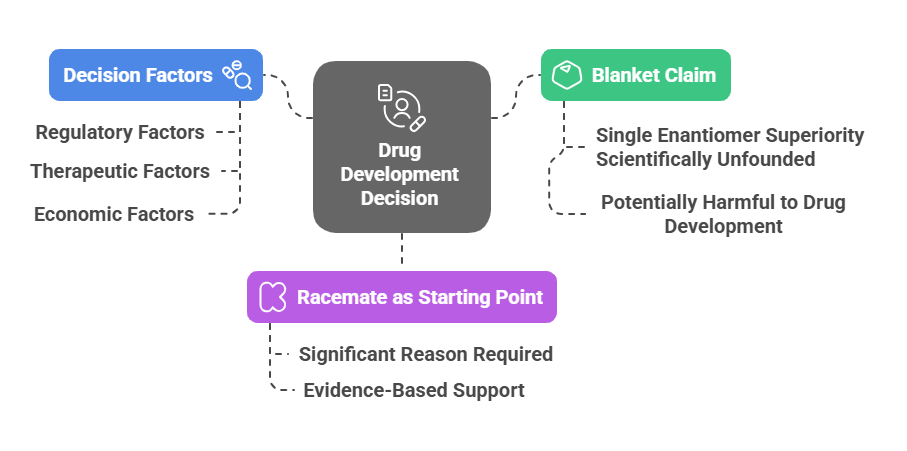
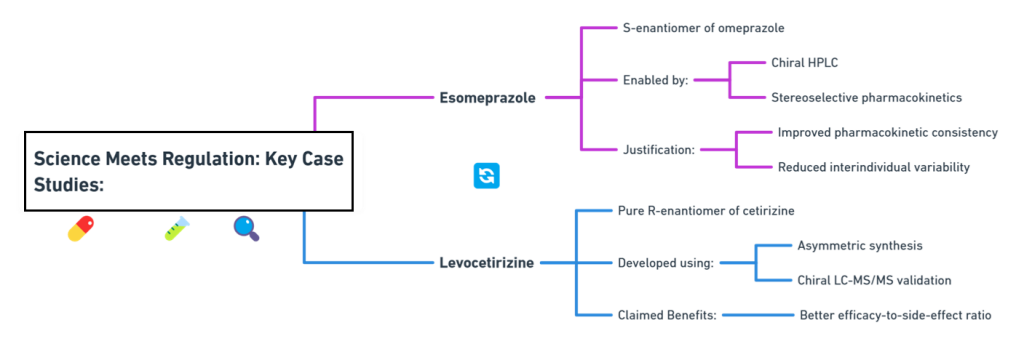
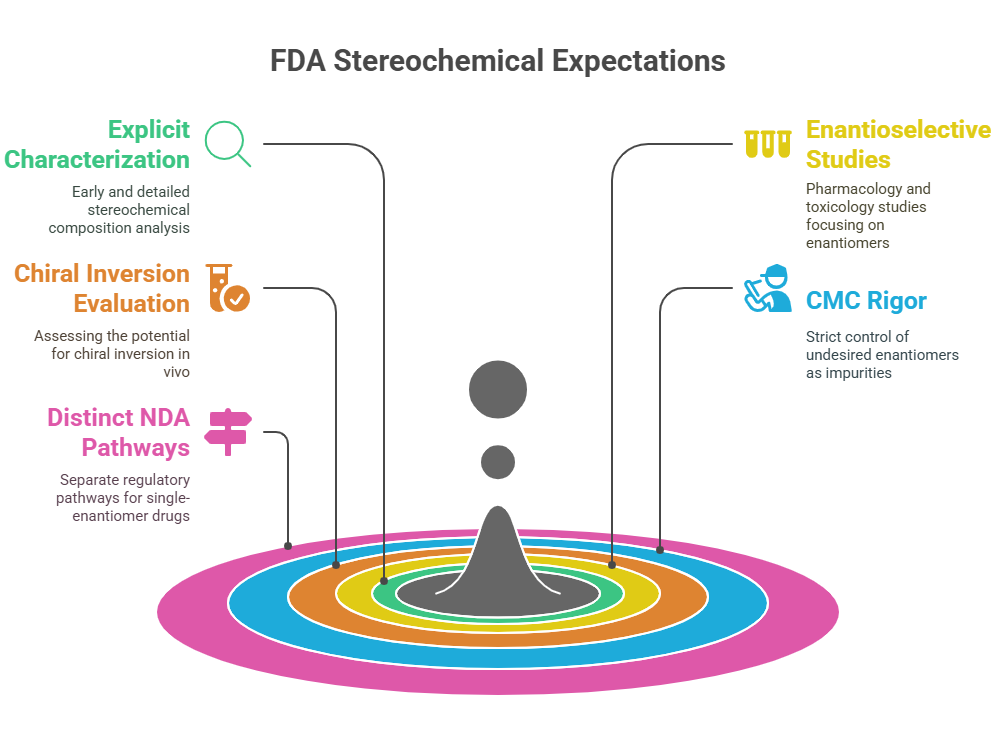
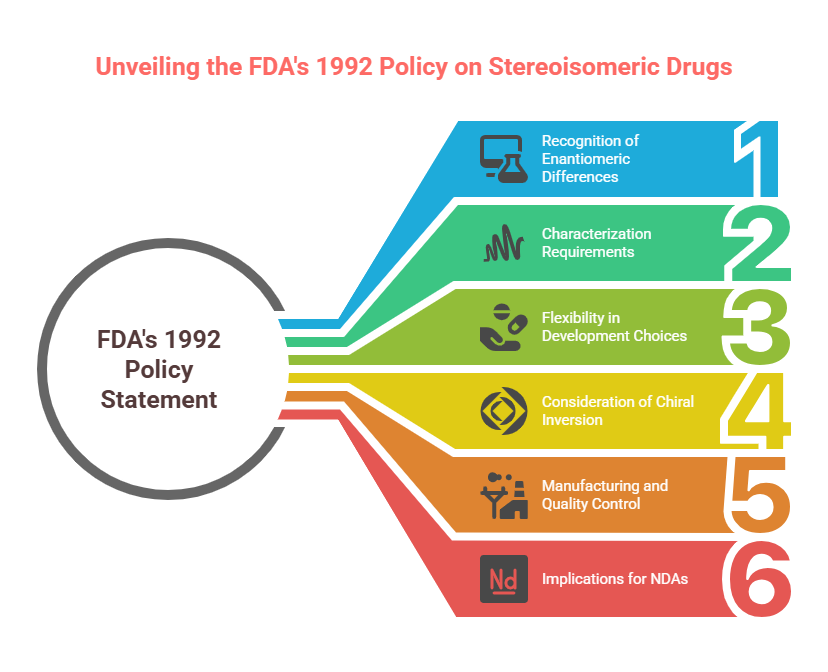
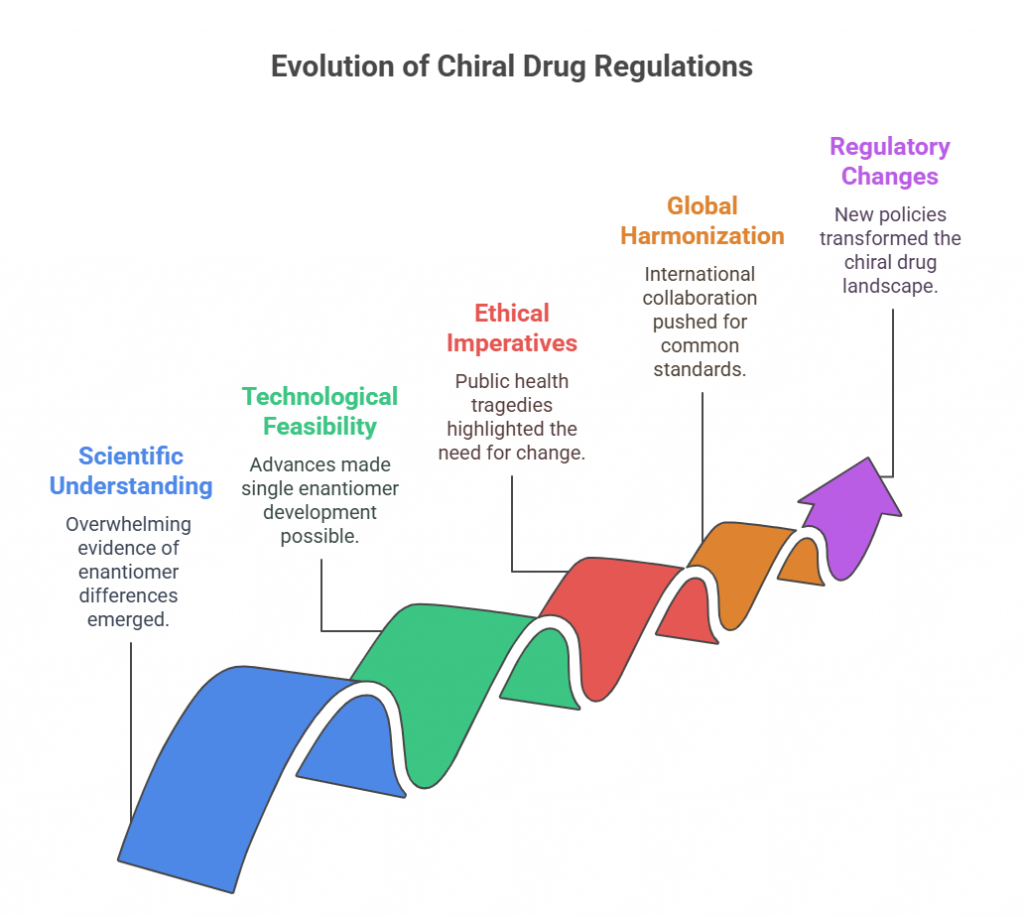
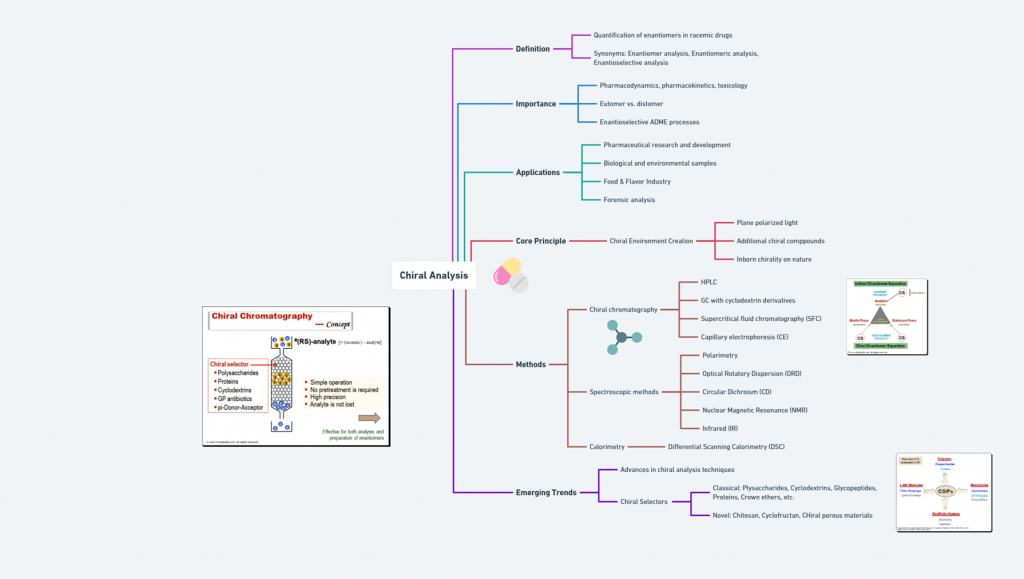
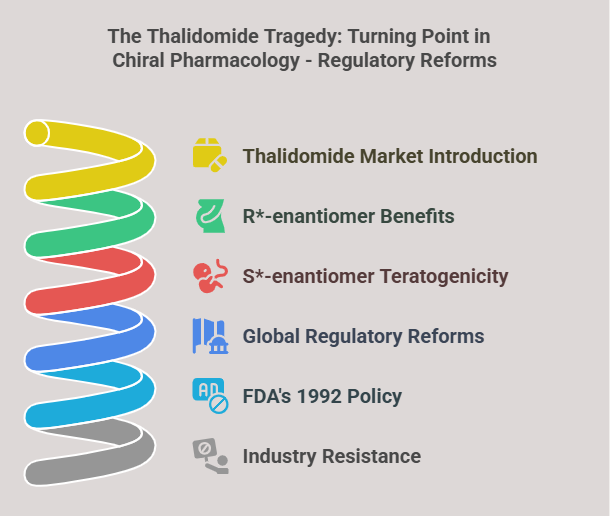
Tagline: “When two mirror images offer different paths, how do regulators choose which one should become medicine?” Introduction The rise of regulatory consciousness around chiral drugs in the 1990s opened a crucial new question:If mirror-image molecules behave differently, how should the pharmaceutical industry -and regulators -decide which enantiomer to develop, approve, and market? This episode explores how regulatory agencies like the FDA, EMA, PMDA, CDSCO, and TGA crafted expectations for the development, approval, and control …
Episode 5: Choosing the Right Twin: Regulatory Expectations for Single Enantiomers Read More »