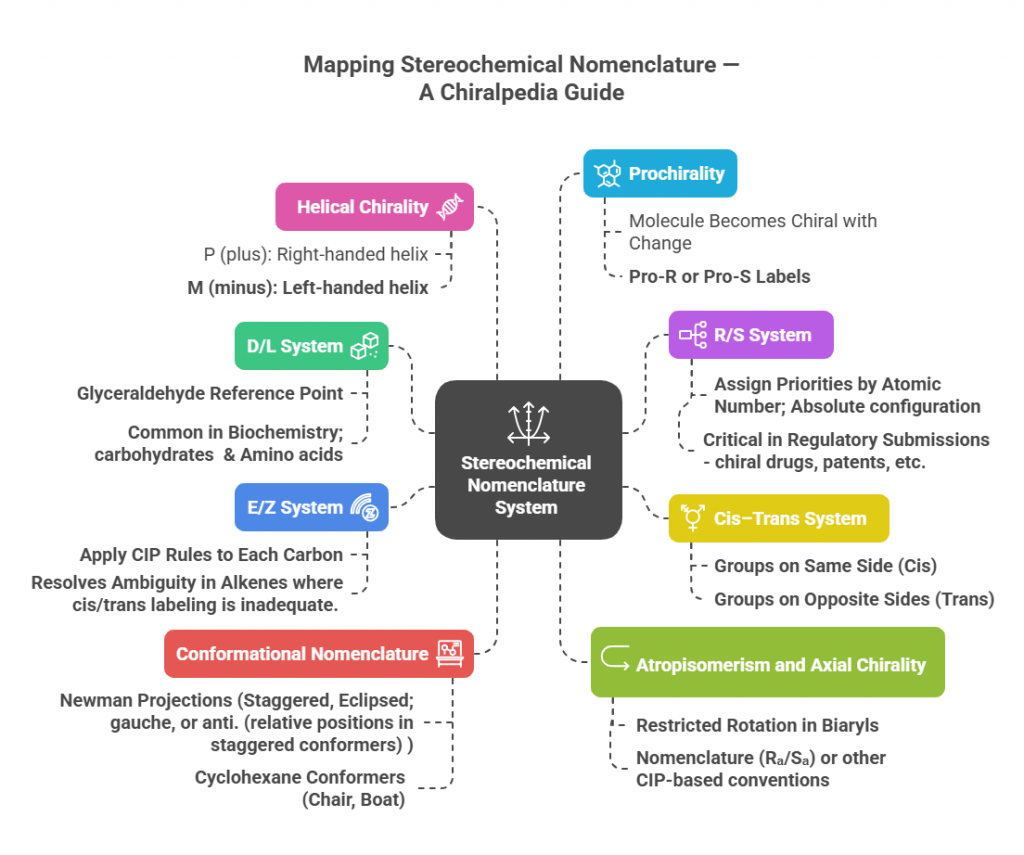
Stereochemistry, the study of spatial arrangements of atoms in molecules, demands a precise and universally accepted nomenclature system. Unlike simple chemical formulas, which only indicate connectivity, stereochemical nomenclature conveys three-dimensional information essential for understanding molecular behavior, biological interactions, and pharmaceutical effects. Several systems have been developed to capture these subtle but critical differences.
🔬✨ Stereochemical Nomenclature System — Now in a Visual Story!
Stereochemical naming systems are often tucked away in textbooks 📚, dense and sometimes intimidating. The goal of this blog is to make them less scary by putting the key concepts into a visual format — a mind map 🧠 that helps you grasp the vocabulary with ease.
🔬 “For an in-depth look at stereochemical nomenclature, check out our blog series: #naming_system.” and references therein
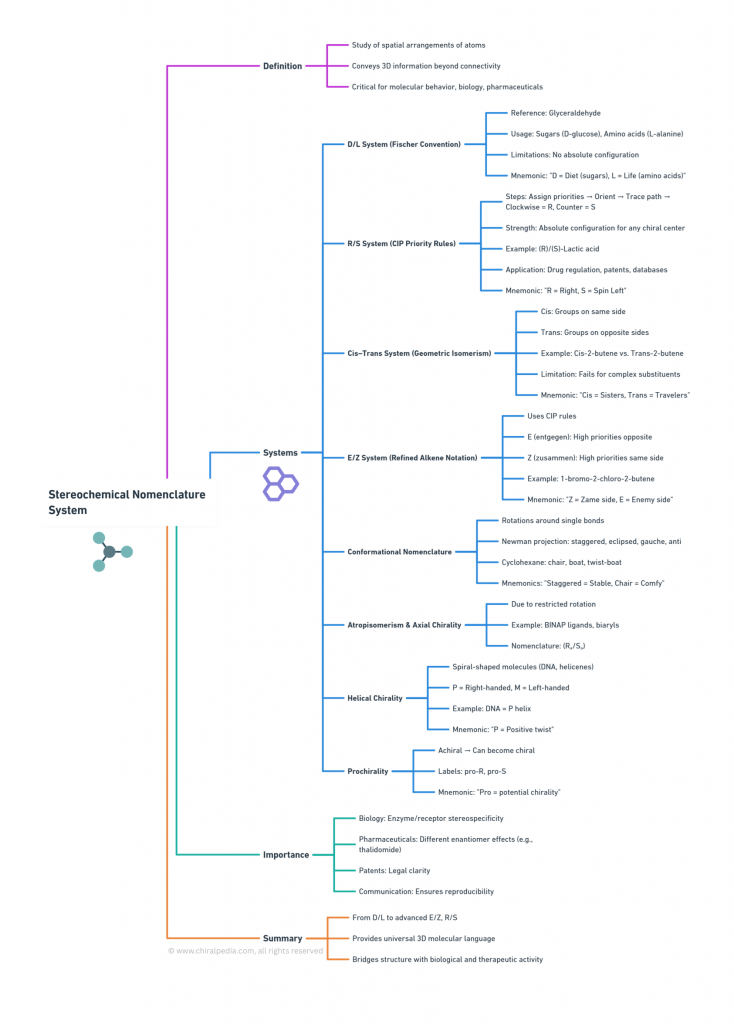
From the early D/L notation to the more rigorous R/S and E/Z conventions—serve as indispensable tools for chemists. They translate flat, two-dimensional chemical formulas into the true three-dimensional architecture of molecules, enabling accurate communication of molecular identity, biological function, and therapeutic safety. For biomolecules, these stereochemical descriptors are not merely labels; they capture functional nuances and evolutionary significance, as enzyme specificity and biological recognition often hinge on subtle stereochemical features.
1. The D/L System (Fischer Convention)
One of the earliest stereochemical naming methods, the D/L system, was introduced in the 19th century to describe sugars and amino acids.
- Reference point: Glyceraldehyde served as the standard. Molecules structurally related to D-glyceraldehyde are labeled D, while those related to L-glyceraldehyde are L.
- Usage: Common in biochemistry to describe natural building blocks like D-glucose or L-alanine.
- Example: Most natural sugars (like glucose) are D, while natural amino acids are usually L.
- Limitations: The system does not indicate the absolute configuration (3D shape) of chiral centers and can be confusing when applied beyond simple biomolecules.
- 💡 Mnemonic: “D for Diet (sugars), L for Life (amino acids).”
2. The R/S System (Cahn–Ingold–Prelog Priority Rules)
The CIP system, developed by Cahn, Ingold, and Prelog, is the most widely adopted for modern stereochemical nomenclature.
- Stepwise method:
- Assign priorities to substituents based on atomic number.
- Orient the molecule so the lowest priority group points away.
- Trace a path from highest to lowest priority.
- Clockwise = R (rectus), counterclockwise = S (sinister).
- Strength: Provides absolute configuration applicable to any chiral center, independent of reference molecules.
- Example: In lactic acid, the –OH group’s orientation decides whether it is (R)- or (S)-lactic acid.
- Application: Critical in regulatory submissions for chiral drugs, patents, and chemical databases.
- 💡 Mnemonic: “R = Right (clockwise).”; “S = Spin Left (counterclockwise).”
3. The Cis–Trans System (Geometric Isomerism)
- What it is: A simple way to describe the geometry of double bonds or ring substituents.
- Cis: Identical (or similar) groups on the same side of a double bond or ring.
- Trans: Identical (or similar) groups on opposite sides.
- Example: Cis-2-butene vs. Trans-2-butene.
- Limitation: Works well for simple cases, but fails when all four substituents on a double bond are different.
- 💡 Mnemonic: “Cis = Sisters stay together.”; “Trans = Travelers go across.”
4. The E/Z System (Alkene Geometry) – (Refined Double Bond Notation)
For double bonds, stereochemical descriptors cis/trans are often insufficient. The E/Z nomenclature is based on the CIP priority rules.
How it works:
- Apply CIP rules to each carbon of the double bond.
- E (entgegen): High-priority substituents on opposite sides of the double bond.
- Z (zusammen): High-priority substituents on the same side.
- Example: 1-bromo-2-chloro-2-butene → can be assigned as E or Z depending on group priorities.
- Significance: Resolves ambiguity in substituted alkenes where cis/trans labeling is inadequate.
- 💡 Mnemonic: “Z = Zame side”; “E = Enemy side.”
5. Conformational Nomenclature
Some molecules can rotate around single bonds, giving different conformations. Such molecules with rotational flexibility require additional descriptors:
- Newman projections classify conformations as staggered, eclipsed; gauche, or anti. (relative positions in staggered conformers)
- Cyclohexane conformers are described as chair, boat, twist-boat, etc.
- Axial and equatorial positions are important in stereochemical stability and reactivity.
- 💡 Mnemonic: “Staggered = Stable, Eclipsed = Energy high.”; “Chair = Comfy, Boat = Wobbly.”
6. Atropisomerism and Axial Chirality
Not all chirality comes from tetrahedral centers. Some molecules are chiral due to hindered rotation rather than tetrahedral centers.
- Restricted rotation in biaryls (like BINAP ligands) creates axial chirality. BINAP ligands and certain biaryls exhibit axial chirality.
- Nomenclature may use (Rₐ/Sₐ) or other CIP-based conventions.
7. Helical Chirality
Some molecules are shaped like spirals. Helical molecules, such as DNA or helicenes. Classified using P (plus) and M (minus)
- P (plus): Right-handed helix.
- M (minus): Left-handed helix.
- Example: DNA is a right-handed (P) helix.
- 💡 Mnemonic: “P = Positive twist (right-handed).”; “M (minus): Left-handed helix.”
8. Prochirality
- A molecule may be achiral now but can become chiral if one substituent changes.
- The positions are labelled pro-R or pro-S to indicate potential stereochemistry.
- 💡 Mnemonic: “Pro- means ‘potential’ chirality — like a rookie waiting to become a pro.”
Importance of Stereochemical Nomenclature
- In biology: Enzymes and receptors are stereospecific — they only “fit” the correct enantiomer.
- Pharmaceutical relevance: Different enantiomers of a drug may have drastically different pharmacological or toxicological profiles (e.g., thalidomide).
- Patent clarity: Legal definitions of molecular identity rely on precise stereochemical naming.
- Communication in science: Accurate stereochemical descriptors ensure reproducibility and avoid misinterpretation.
Summary
Together, the full suite of stereochemical systems—D/L, R/S, cis–trans, E/Z, conformers, axial and helical chirality, and prochirality—provides a universal language to describe molecular geometry in three dimensions. Far more than a naming convention, this framework acts as a critical bridge linking chemical structure with real-world biological activity and pharmaceutical performance.
Further Reading
- Fischer Projection: hassle free way to depict a stereoformula in 2D projection
- Naming enantiomers: the left-(or right-) handed?
- Cis-trans and E-Z notation: choose your side
- The meso compounds: finding plane of symmetry
- Erythro- and Threo- prefixes: the (same-) or (opposite-) side?
- Atropisomers: things are tight, single bond won’t rotate
- D-/L- system naming: the (left-) or (right-) hand side?