“Chloramphenicol possesses two chiral carbon atoms in the acylamidopropanediol chain. Biological activity resides almost exclusively in the D-threo-, isomer: the L-threo–and the D– and L-erythro– isomers are virtually inactive.” – – – from “Wilson and Gisvold’s Textbook of organic medicinal and pharmaceutical chemistry”.
In the name “D-threo– and L-threo-Chloramphenicol”, what does the prefix threo– and erythro– in the nomenclature mean? How do we translate the name to structure? This blog is basically to understand and interpret the erythro-/threo- prefixes employed while naming organic molecules.
The prefixes erythro- and threo– nomenclature originates from the four carbon sugars erythrose and threose. In a molecule containing n stereocenters, the number of possible stereoisomers vary depending whether the molecule is constitutionally unsymmetrical (non-identical centers of chirality) or constitutionally symmetrical.
Constitutionally symmetrical and unsymmetrical molecules
In a constitutionally symmetrical molecule, the chiral/stereogenic centers equidistant from the geometrical center of the molecule are identically substituted. In molecule (I), 2,3-diibromosuccinic acid, contains two chiral centers but the four ligands attached to one carbon atom (COOH, Br, H and CH(Br)COOH are the same as those attached to the second chiral center. Hence the molecule (I) is categorized as constitutionally symmetrical.
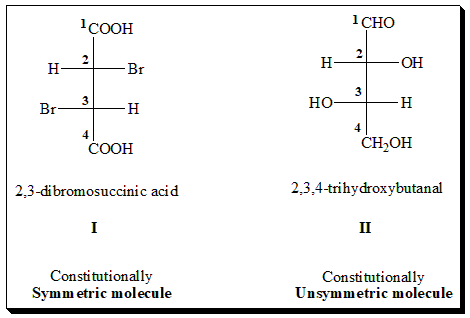
On the other hand in constitutionally unsymmetrical molecule, the chiral centers equidistant from the geometrical center of the molecule are not identically substituted. For instance in the case of molecule (II), 2,3,4-trihydroxybutanal (erythrose), has again two chiral centers but the four ligands attached to one carbon atom are (CHO, H, OH and CH(OH)CH2OH) and on the other chiral center are (CH2OH, OH, H and CH(OH)CHO, which is different. Here we find the molecule has two non-identically substituted chiral centers making the molecule (II) constitutionally unsymmetrical.
To understand the erythro-/threo– nomenclature let us examine constitutionally unsymmetrical stereogenic center.
Constitutionally unsymmetrical stereogenic center
An acyclic, constitutionally unsymmetrical molecule can exist in 2n stereoisomers which are enantiomeric in pairs. In other words, such a molecule can exhibit 2 (n-1) enantiomeric pairs. As a simple example with n⹀2, let us examine four carbon sugars, with two different stereogenic centers. The C4 sugar therefore, with two different stereogenic centers, should exist in four enantiomeric forms and two racemic mixtures. These are known as threose and erythrose. and have the Fischer projections III-VI.
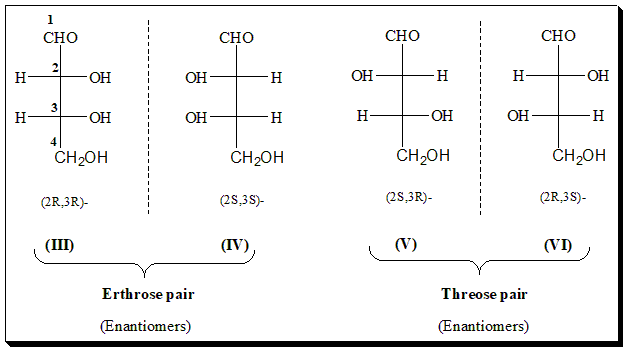
Erythrose exist in enantiomeric forms (III and IV) as does threose (V and VI). Between any erythro-isomer and threo-isomer, the relationship is that of diastereoisomerism. Thus compounds represented by (III and V) and (IV and VI) are not mirror-images; are diastereoisomeric (2n -2 diastereomers). These diastereomers which differ in the configuration of a single stereocenter (i.e., which have identical configuration on n-1 centers) are called epimers. Any stereoisomer in such a series has n epimers.
The prefixes threo- and erythro-
Other molecules with two different stereogenic centers, not necessarily on adjacent carbon atoms, are frequently designated by the prefix threo- and erythro-. These are interpreted as meaning that two identical (or similar groups are on the same side (erythro–) or on opposite side (threo–) of the carbon chain when expressed as a Fischer projection.
Case study: Chloramphenicol Stereoisomers (in Fischer Projection)
The broad spectrum antibacterial Chloramphenicol is a good example to illustrate the erythro-/threo notation. Chloramphenicol contains two stereogenic centers and can exist in four stereoisomeric forms. These are presented in the Fischer projection formula (VII, VIII, IX, & X).
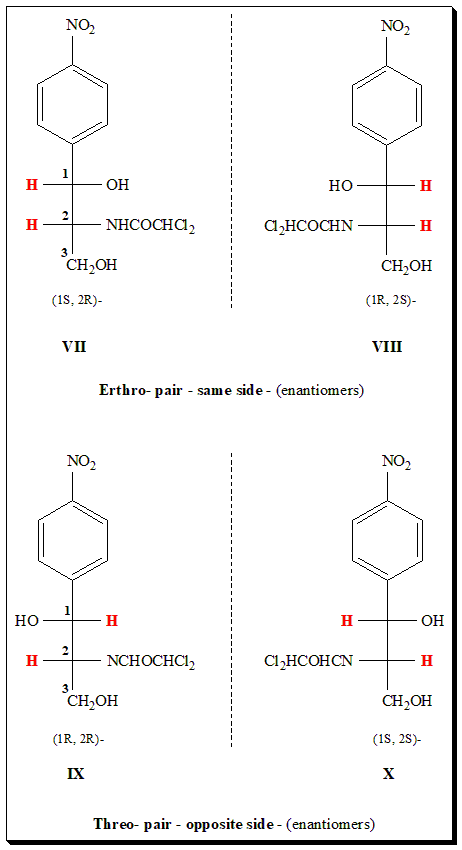
Among the four stereoisomers of chloramphenicol the biological activity resides only in the D-threo-isomer (IX) with absolute configuration (R,R). The IUPAC name for chloramphenicol is 2,2-dichloro-N-[(1R,2R)-1,3-dihydroxy-1-(4-nitrophenyl)propan-2-yl]acetamide
Practice exercise
- Apply prefixes, threo– or erythro– correctly to the following structures [XI, XII, and XIII]
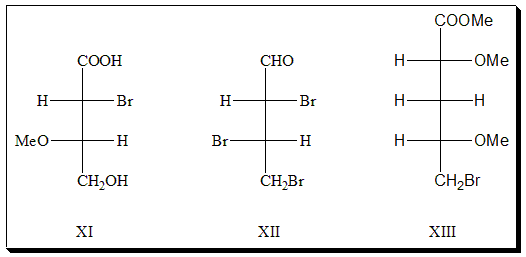
2. Draw Fischer projection formula of Ephedrine, a CNS stimulant, which carries two stereogenic centers. Identify the erythro-and threo- pairs and study the stereoisomeric relationships between the pairs. Comment on their biological activity.
References and Further reading
Wilson and Gisvold’s Textbook of organic medicinal and pharmaceutical chemistry, Edited by John H. Block and John M. Beale, Jr., 12th edition, Lippincott Williams & Wilkins, New York, 2010.
Foye’s Principles of Medicinal Chemistry, Lemke, Thomas L. (Editor), Williams, David A. (Editor), Roche, Victoria F. (Editor), 7th edition, Lippincott Williams & Wilkins, New York, 2012.
Jerry March, Advanced Organic Chemistry: Reactions, Mechanisms and Structure, John Wiley & Sons Inc., New York, 2006.
Bernard Testa, Principles of organic stereochemistry, Marcel Dekker Inc., New York, 1979.