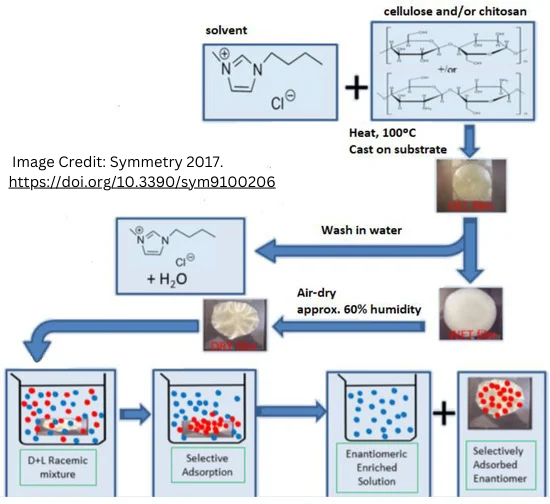
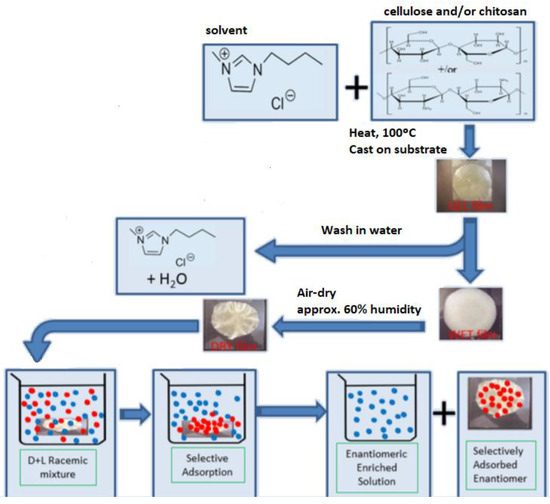
Lead
Chiral resolution techniques have long been a cornerstone in pharmaceutical and chemical industries, where separating enantiomers is crucial for producing safe and effective products. Among the most promising methods are membrane-based and kinetic resolution techniques, both of which have seen significant advancements in recent years. These methods are becoming increasingly relevant as industries seek more efficient, sustainable, and cost-effective approaches to chiral separation.
1. Overview of Membrane and Kinetic Techniques
Membrane and kinetic resolution techniques offer two distinct yet complementary methods for chiral separation. A comparative analysis is presented in a visual format for easy understanding.
Membrane-based separation uses selective membranes that allow certain enantiomers to pass through while blocking others, effectively separating a racemic mixture based on molecular size or affinity. This method is highly appealing due to its efficiency, low energy consumption, and potential for continuous operation, making it suitable for large-scale industrial applications (Suliman, 2023).
Kinetic resolution, on the other hand, separates enantiomers based on differences in their reaction rates in the presence of a chiral catalyst. This method exploits the fact that one enantiomer reacts faster than the other under specific conditions, allowing selective conversion of one enantiomer while the other remains unreacted (Malacarne, 2024). As kinetic resolution is often used in conjunction with catalytic processes, it holds great promise for high-throughput industrial applications, particularly in the production of pharmaceuticals.
2. The Rising Importance of Sustainable and Energy-Efficient Methods
The rising demand for sustainable manufacturing processes has spurred interest in membrane and kinetic resolution techniques. Traditional methods of chiral resolution, such as crystallization and chromatography, can be resource-intensive and generate significant waste. In contrast, membrane-based methods require less solvent and energy, and they produce fewer byproducts. This makes them an attractive option for industries aiming to reduce their environmental footprint.
Membrane separation also offers the potential for continuous processing, further enhancing its energy efficiency. By operating at ambient temperatures and pressures, membrane-based techniques minimize the need for extensive heating or cooling, leading to significant reductions in energy consumption. This is particularly important in large-scale pharmaceutical production, where sustainability is becoming a key factor in process design.
Kinetic resolution, while slightly more complex, also aligns with the principles of green chemistry. The use of catalysts in kinetic resolution allows for highly selective reactions that generate fewer byproducts, making the process more atom-efficient. In addition, the ability to recycle catalysts further reduces waste, making kinetic resolution an appealing choice for industries focused on sustainability. As environmental regulations become more stringent, the demand for sustainable chiral resolution methods will continue to grow, pushing membrane and kinetic techniques to the forefront of innovation.
2. Membrane-Based Chiral Resolution
Principles of Membrane Separation
Membrane-based chiral resolution operates on the principle of selective permeability, where membranes are designed to allow one enantiomer to pass through while retaining the other. This separation is typically achieved by incorporating chiral selectors into the membrane material, which interact differently with each enantiomer. These interactions can be based on size exclusion, charge, or molecular recognition mechanisms.
The effectiveness of membrane separation largely depends on the membrane’s material and structure. The membranes used in chiral separation can be either solid or liquid, each with distinct advantages depending on the application. Membranes are particularly useful in continuous flow systems, where large volumes of material can be processed without interruption. This makes them an ideal choice for industries that require high-throughput chiral resolution.
Types of Chiral Membranes: Liquid vs Solid
Chiral membranes can be broadly classified into two types: liquid membranes and solid membranes. Liquid membranes consist of a liquid phase held between two immiscible liquid phases, where the selective transport of enantiomers occurs. The major advantage of liquid membranes is their ability to transport specific enantiomers through facilitated diffusion, making them suitable for small-scale, high-precision applications. However, they can suffer from stability issues due to the volatility of the liquid phase.
Chiral Liquid Membrane
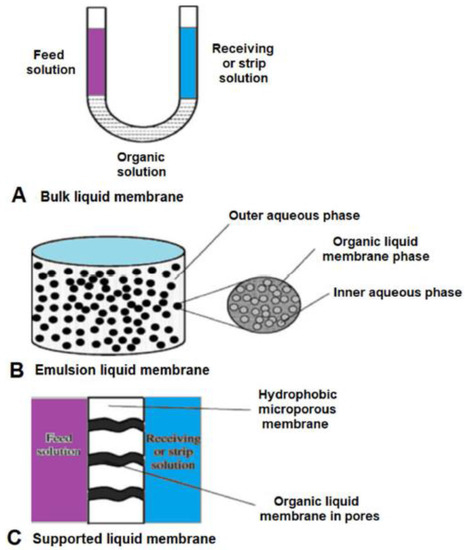
Liquid membrane types: emulsion, bulk, and supported.
Emulsion Liquid Membrane: This type offers good separation but is complex to operate and prone to leaks. It involves creating a double emulsion, where an aqueous phase with a chiral carrier is mixed with an organic phase, then dispersed in another aqueous phase with the racemic mixture. The high interfacial area is a plus, but stabilizing and breaking the emulsions requires extra compounds.
Bulk Liquid Membrane: This type is stable but has a low mass transfer rate. It’s mainly used for preliminary tests due to its simplicity and low reproducibility. It provides a low interfacial area, making it less effective for detailed studies.
Supported Liquid Membrane: Gaining popularity for its ease of scaling up and low operating costs, this type holds liquid in support pores by capillary forces, allowing transport between source and receiving phases. It’s more suitable for industrial applications and has been used in pharmaceutical separations. It requires only a small amount of chiral extractant for high productivity. However, it lacks long-term stability as the solvent can evaporate or the transporter complex can wash out during operation.
Chiral Solid Membrane
Solid membranes, in contrast, are composed of polymers or other solid materials that are functionalized with chiral selectors. These membranes are more robust and suitable for large-scale industrial applications. Solid membranes can be designed with various pore sizes and structures, enabling selective permeability based on the molecular size or interaction strength with the chiral selector. Advances in solid membrane technology have made them more efficient, scalable, and adaptable to various chiral compounds).
Types of Enantioselective solid membranes: inherent chiral membranes and membranes with immobilized chiral selectors.
Inherent Chiral Membranes: These are made by casting solutions of chiral polymers, which have chiral backbones or side chains. Common materials include poly(-methyl- L-glutamate), alginate, chitosan, and cellulose. The enantioselectivity comes from chiral carbons in the main chain. However, achieving high efficiency can be challenging when enantiomers interact with flexible side chains that lack crucial chiral sites.
Functionalized Membranes: These membranes are enhanced with immobilized chiral selectors to improve enantioselectivity. Modified polymers with chiral branches, such as chiral metal-Schiff base complexes or saccharide side chains, are used. Other examples include membranes made from acetylated beta-cyclodextrin-functionalized cellulose or polysulfone grafted with N-dodecyl-4®-hydroxy-L-proline. Chiral selectors like beta-cyclodextrin, crown ethers, proteins, and DNA can be incorporated.
3. Kinetic Resolution
Introduction to Kinetic Resolution and its Mechanisms
Kinetic resolution is a highly selective method of chiral separation that relies on the differential reaction rates of enantiomers in the presence of a chiral catalyst. In a racemic mixture, one enantiomer reacts faster than the other, allowing for the preferential conversion of the more reactive enantiomer into a product, while the slower-reacting enantiomer remains unchanged. This selective conversion enables the isolation of the unreacted enantiomer with high purity.
The efficiency of kinetic resolution depends on the reaction kinetics and the choice of catalyst. Chiral catalysts play a critical role in ensuring that the reaction proceeds at different rates for the two enantiomers. The use of catalysts not only increases the reaction rate but also enhances the selectivity of the process, making kinetic resolution an attractive option for large-scale industrial applications.
Role of Catalysts in Enantioselective Reactions
Catalysts are the driving force behind kinetic resolution. The selection of an appropriate chiral catalyst is crucial, as it determines the enantioselectivity and efficiency of the reaction. Common catalysts used in kinetic resolution include transition metal complexes, enzymes, and organocatalysts. Each type of catalyst offers distinct advantages depending on the reaction conditions and the specific enantiomers involved.
For example, enzymatic catalysts are widely used in kinetic resolution due to their high specificity and mild reaction conditions. Enzymes such as lipases and esterases can selectively hydrolyze one enantiomer in a racemic mixture, leaving the other unreacted. Similarly, organocatalysts are gaining popularity in kinetic resolution for their ability to catalyze a wide range of reactions with high enantioselectivity, often without the need for metals, making them more environmentally friendly.
4. Applications and Advantages
How Membrane Separation is Used in Large-Scale Production
Membrane-based chiral resolution is increasingly being adopted for large-scale production in industries such as pharmaceuticals, agrochemicals, and food processing. The scalability of membrane technology, combined with its low energy consumption and continuous operation capabilities, makes it a viable solution for high-throughput applications. In the pharmaceutical industry, membrane separation has been used to produce enantiopure compounds on an industrial scale, ensuring the high purity required for regulatory approval.
In addition, membrane systems can be easily integrated into existing production processes, reducing the need for extensive reconfiguration of manufacturing plants. The ability to operate at ambient conditions and avoid the use of harsh chemicals also makes membrane separation an environmentally friendly option, further contributing to its adoption in sustainable industrial practices.
Advantages of Kinetic Resolution in High-Throughput Settings
Kinetic resolution offers several advantages in high-throughput industrial settings. Its ability to achieve high enantioselectivity with minimal steps makes it an efficient process for large-scale production. The use of catalysts, particularly enzymes, allows for reactions to occur under mild conditions, reducing the need for excessive energy input and minimizing the risk of unwanted side reactions.
Additionally, kinetic resolution can be applied to a wide range of substrates, making it a versatile tool for the synthesis of complex drug intermediates. The process can also be optimized to achieve higher yields and greater purity, reducing the need for additional purification steps and lowering production costs.
Sustainability and Cost-Efficiency of Both Methods
Both membrane and kinetic resolution techniques offer significant advantages in terms of sustainability and cost-efficiency. Membrane-based methods require fewer solvents and less energy compared to traditional separation techniques, making them a more environmentally friendly option. The ability to operate continuously also reduces downtime and increases overall production efficiency.
Kinetic resolution, with its reliance on catalytic processes, is similarly sustainable. The use of recyclable catalysts and the ability to minimize waste through selective reactions align with the principles of green chemistry, reducing the environmental impact of industrial-scale production. Both methods are poised to play an important role in the shift toward more sustainable and cost-efficient manufacturing practices.
5. Challenges and Future Directions
Limitations of Membrane and Kinetic Resolution
Despite their many advantages, membrane and kinetic resolution techniques are not without limitations. One of the primary challenges associated with membrane-based chiral resolution is the development of membranes with sufficient selectivity and stability. While solid membranes are robust and suitable for industrial-scale applications, they can suffer from fouling, which reduces their efficiency over time. Liquid membranes, while more selective, are prone to leakage and degradation, limiting their scalability.
Kinetic resolution, on the other hand, is limited by the fact that it can only achieve a maximum theoretical yield of 50%, as only one enantiomer is selectively converted in a racemic mixture. This means that additional steps, such as recycling or racemization of the unreacted enantiomer, are often necessary to improve overall yield (Pinto, 2020). Additionally, the cost and availability of chiral catalysts can be a limiting factor, particularly for complex reactions requiring highly specialized catalysts.
How These Methods Are Evolving to Meet Industrial Demands
To address these challenges, significant research is being conducted to improve both membrane and kinetic resolution techniques. In membrane technology, advances in materials science are leading to the development of more durable and selective membranes, such as those incorporating metal-organic frameworks (MOFs) and covalent organic frameworks (COFs), which offer enhanced performance and stability.
In kinetic resolution, efforts are being made to develop more efficient catalysts that can achieve higher yields and selectivity. The use of enzymatic catalysts in particular is gaining traction, as they offer high enantioselectivity and can be recycled, reducing costs and improving sustainability. These innovations are helping to push membrane and kinetic resolution techniques to the forefront of industrial chiral separation.
6. Conclusion
The Future of Kinetic and Membrane Resolution in Pharmaceuticals
As industries continue to prioritize sustainability, efficiency, and cost-effectiveness, membrane and kinetic resolution techniques are set to become increasingly important in the pharmaceutical and chemical sectors. Both methods offer significant advantages over traditional separation techniques, particularly in terms of scalability and environmental impact. Advances in materials science and catalysis are helping to overcome the current limitations of these techniques, ensuring that they remain viable options for large-scale chiral separation in the future.
With the growing demand for enantiopure compounds, particularly in the pharmaceutical industry, the development and optimization of membrane and kinetic resolution techniques will be key to meeting industrial needs. As research continues to push the boundaries of what these methods can achieve, we can expect to see even greater innovation in the field of chiral separation, driving progress toward more sustainable and efficient manufacturing processes.
Further Reading
Suliman, A. (2023). Emerging Developments in Separation Techniques and Analysis of Chiral Pharmaceuticals. Molecules, 28(5), pp.1-12.
Malacarne, F. (2024). Unconventional Approaches for Chiral Resolution. Analytical and Bioanalytical Chemistry, 416, pp.3677-3685.
Pinto, M. (2020). Chiral Separations in Preparative Scale: A Medicinal Chemist’s Viewpoint. Journal of Medicinal Chemistry, 63(14), pp.7889-7899.
Sui, H. (2023). Strategies for Chiral Separation: From Racemate to Enantiomer. Royal Society of Chemistry.
Suliman, A. (2023). Emerging Developments in Separation Techniques and Analysis of Chiral Pharmaceuticals. Molecules, 28(5), pp.1-12.
Fernandes, C.; Tiritan, M.E.; Pinto, M.M.M. Chiral Separation in Preparative Scale: A Brief Overview of Membranes as Tools for Enantiomeric Separation. Symmetry 2017, 9, 206. https://doi.org/10.3390/sym9100206
Capdevila-Echeverria, J., Wang, J., Lakerveld, R., & ter Horst, J. T. (2021). Process modeling and optimization of continuous chiral resolution by integration of membrane and crystallization technologies. Journal of Membrane Science. https://doi.org/ 10.1016/j.memsci.2021.119359
Wang, F., He, K., Wang, R., Ma, H., et al. (2024). A Homochiral Porous Organic Cage-Polymer Membrane for Enantioselective Resolution. Advanced Materials. https://doi.org/10.1002/adma.202400709