Ibuprofen is a widely used non-steroidal anti-inflammatory drug (NSAID). It is a monocarboxylic acid that is propionic acid in which one of the hydrogens at position 2 is substituted by a 4-(2-methylpropyl)phenyl group. Hence belongs to the propionic acid derivative class of NSAIDs. It is commonly prescribed for pain, inflammation, and fever. Available as over-the-counter (OTC) and prescription medication worldwide.
Chirality and Biological Activity
Structurally, Ibuprofen is a chiral molecule with one stereogenic center at the α-position to the carboxylic acid group. Ibuprofen exists as two enantiomers: (S)-ibuprofen and (R)-ibuprofen. The marketed form is a racemic mixture (1:1 of both enantiomers). Only the (S)-enantiomer exhibits significant pharmacological activity by inhibiting the cyclooxygenase (COX-1 and COX-2) enzymes, reducing prostaglandin synthesis. Dexibuprofen, (the (S)-enantiomer), a chiral switch, has been developed which can provide the same therapeutic effect at lower doses, potentially reducing adverse effects.
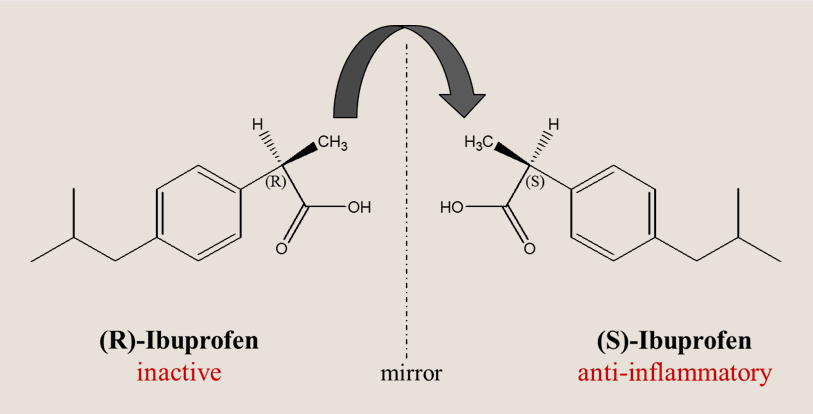
The (S)-enantiomer of ibuprofen is pharmacologically active and responsible for the desired therapeutic effects through inhibition of COX enzymes. The (R)-isomer, though inactive toward COX, undergoes a unidirectional, CoA-dependent metabolic inversion in the liver, converting a significant portion into the active (S)-form. Thus, when ibuprofen is administered as a racemate, the inactive distomer partly transforms into the eutomer, while the latter remains unaffected. This in vivo recycling mechanism explains why racemic ibuprofen maintains clinical efficacy and cost-effectiveness, despite the availability of enantiopure formulations. (S)-ibuprofen → responsible for both therapeutic effects and the majority of NSAID-related adverse effects. (R)-ibuprofen → considered pharmacologically inactive but not directly toxic; however, its persistence adds unnecessary drug load
Nomenclature:
(2R)-2-[4-(2-methylpropyl)phenyl]propanoic acid
Exercise
- Why is Ibuprofen considered a chiral drug, and what makes its stereochemistry clinically relevant?
- What is meant by the in vivo chiral inversion of Ibuprofen, and how does it influence the drug’s pharmacological profile?
- Which stereoselective techniques are commonly used to separate or analyze Ibuprofen enantiomers?
Therapeutic category
Nonsteroidal Anti-inflammatory drug. The mechanism of action of ibuprofen is as a Cyclooxygenase Inhibitor.
Further Reading
Petrie, B., Camacho-Muñoz, D. Analysis, fate and toxicity of chiral non-steroidal anti-inflammatory drugs in wastewaters and the environment: a review. Environ Chem Lett 19, 43–75 (2021). https://doi.org/10.1007/s10311-020-01065-y
Sanins SM, Adams WJ, Kaiser DG, Halstead GW, Baillie TA. Studies on the metabolism and chiral inversion of ibuprofen in isolated rat hepatocytes. Drug Metab Dispos. 1990 Jul-Aug;18(4):527-33. PMID: 1976078.
Chiral switch. Wikipedia, Wikipedia Foundation, 30/07/2022. https://en.wikipedia.org/wiki/Chiral_switch