“Approving a drug is only half the journey. The true story unfolds where science, ethics, commerce, and access collide.”
Introduction
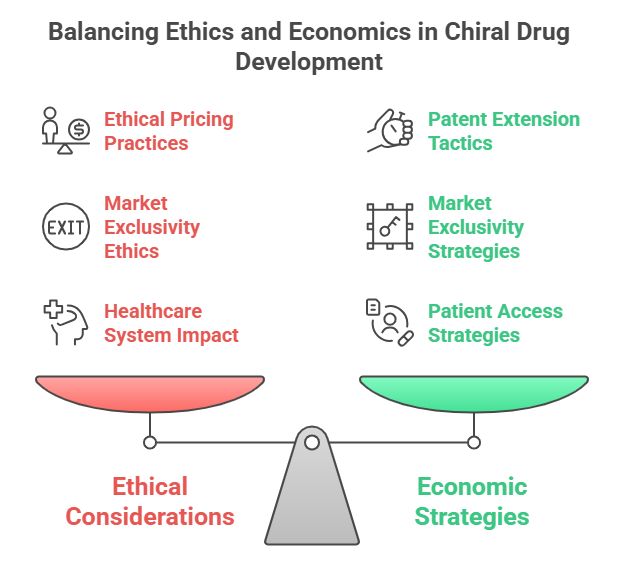
Chirality reshaped regulatory science and drug development -but its impact extends far beyond the laboratory and approval desk. The commercialization of single-enantiomer drugs, especially through chiral switches, has raised important ethical and economic debates about innovation, access, affordability, and equity. In this episode, we delve into the ethical dilemmas and economic strategies tied to chiral drugs:
- How have chiral drugs been used to extend patents and market exclusivities?
- Are single-enantiomer drugs always priced and marketed ethically?
- What is the real-world impact on healthcare systems and patients?
Beyond the purity of molecules lies a far messier world of decisions, consequences, and responsibilities.
Intellectual Property and Patent Strategies
One of the most striking impacts of chiral awareness has been on intellectual property (IP) strategies in the pharmaceutical industry.
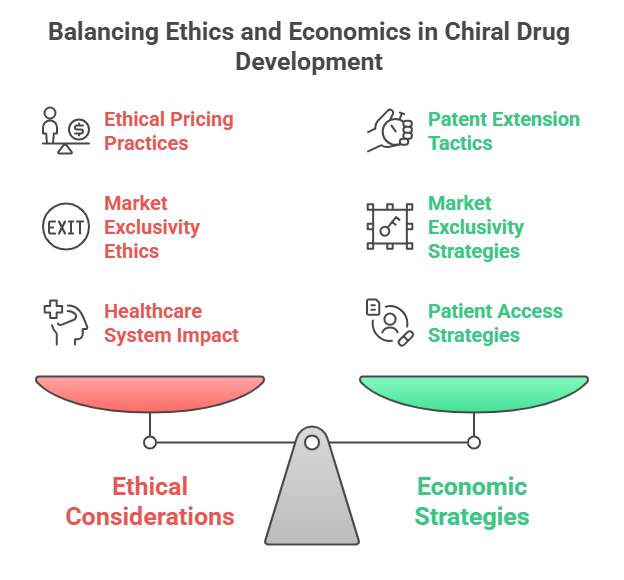
How Chiral Switches Extend Exclusivity
- Racemates patented and marketed → Single enantiomer later patented separately.
- “New drug” designation for the single-enantiomer form allows additional market exclusivity (5 years in U.S. for NMEs).
- Patent linkage (e.g., Orange Book listings) can delay generic entry.
This maneuver -known as evergreening -allows companies to protect revenue streams beyond the original racemate’s patent expiry (Agranat & Wainschtein, 2010).
Famous Examples:
- Esomeprazole (Nexium®) following omeprazole (Prilosec®).
- Escitalopram (Lexapro®) following citalopram (Celexa®).
- Levocetirizine (Xyzal®) following cetirizine (Zyrtec®).
Each case extended the parent drug’s lifecycle through strategic use of chirality and regulatory frameworks.
Economic Impact: Costs and Access
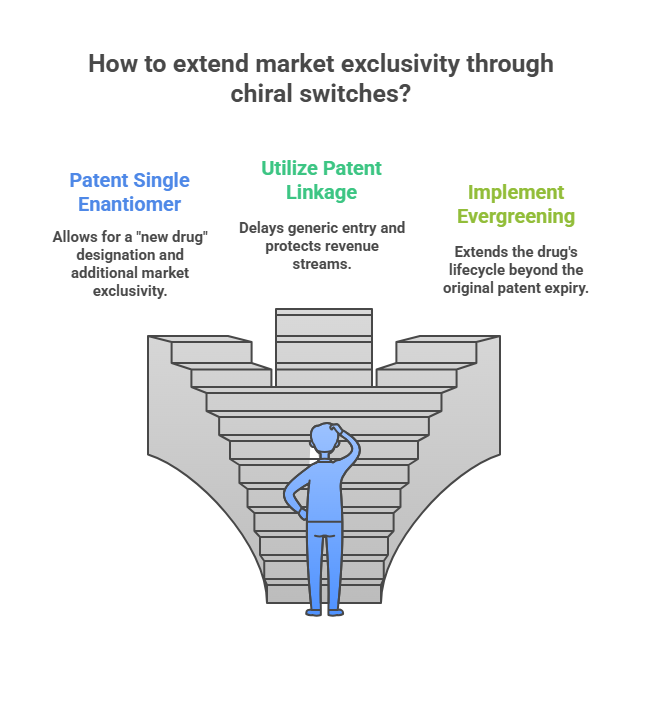
While innovation deserves reward, critics argue that many chiral switches:
- Do not offer proportionally better clinical outcomes to justify higher prices.
- Delay generic competition, keeping prices elevated.
- Contribute to escalating healthcare costs without corresponding public health gains (Hancu & Modroiu, 2022).
Insurance companies and healthcare systems have increasingly resisted covering higher-priced single-enantiomer drugs unless clinical superiority is clearly demonstrated.
For patients, especially in low- and middle-income countries:
- Affordability becomes a barrier when racemates are displaced by costly single-enantiomer alternatives.
- Out-of-pocket expenditure can rise steeply for marginal therapeutic gains.
Thus, the economic dimension of chiral switches demands close scrutiny.
Ethical Dimensions: Innovation vs. Exploitation
The ethics of chiral drug commercialization revolve around several critical tensions:
1. True Innovation or Market Exploitation?
While some switches genuinely improve patient outcomes (e.g., esomeprazole), others provide only marginal benefits.
Ethical question:
- Is it right to seek new patents and higher prices when clinical advantage is minimal?
- Should regulatory frameworks prevent or discourage such practices?
2. Patient Autonomy and Informed Choice
Patients often trust physicians and payers to select therapies in their best interest.
If single-enantiomer drugs are marketed aggressively without full transparency:
- Patient autonomy is undermined.
- Informed consent about drug choices and costs may not be respected.
Transparency around comparative efficacy, side effects, and costs is an ethical imperative.
3. Equity in Access
Wealthier patients or health systems can afford new single-enantiomer formulations; poorer populations often cannot. Thus:
- Health inequities may worsen if essential drugs become costlier without clear clinical necessity.
- Global access gaps widen, particularly for chronic diseases like acid reflux or allergies.
Chirality-driven commercial strategies must consider global health equity, not just regional profitability.
Regulatory Responses to Ethical Challenges
Regulatory agencies have responded to these ethical dilemmas in several ways:
Stricter Approval Standards
- Regulators now require clear clinical superiority over racemates for marketing authorization of single enantiomers.
- Example: FDA’s demand for superiority data in chiral switches submitted as new NDAs (FDA, 1992).
Transparent Labeling
- EMA, FDA, and TGA guidelines require transparent discussion of comparative efficacy and pharmacokinetics in product labeling (EMA, 1994).
Encouragement of Comparative Effectiveness Research
- Agencies and public health bodies encourage studies comparing racemates and single enantiomers to inform payer and prescriber decisions.
Payers and Health Technology Assessments (HTA)
Health systems increasingly rely on Health Technology Assessment (HTA) frameworks to evaluate chiral drugs.
Key questions include:
- Does the single enantiomer significantly improve clinical outcomes?
- Is the incremental cost justified by the benefit?
- What are the broader public health implications of funding the more expensive drug?
HTA agencies like NICE (UK) and CADTH (Canada) have sometimes refused to recommend reimbursement of single-enantiomer drugs when evidence of substantial advantage was lacking.
Industry Defense of Chiral Switches
Pharmaceutical companies argue that:
- Chiral switches often involve substantial investment in new clinical trials, manufacturing validation, and regulatory submissions.
- Patients benefit from reduced side effects, lower doses, and more predictable responses.
- Without incentives, companies may be less willing to optimize existing drugs post-approval.
There is some truth here: innovation must be financially viable. But the ethical issue centers on proportionality -does the patient receive real value for the higher cost?
Patient Advocacy and Public Pressure
Growing awareness of drug pricing issues has led to:
- Greater public scrutiny of “new” drugs that seem like minor tweaks of existing ones.
- Patient advocacy groups demanding better transparency and access.
- Political pressure for drug price regulation, especially in the U.S. and Europe.
Chiral switches thus sit at the intersection of pharmaceutical ethics, public health policy, and economic justice.
Looking Forward: Models for Ethical Chiral Innovation
Several emerging models aim to balance innovation with ethics:
- Early single-enantiomer development: Designing single enantiomers from the start, avoiding later switches.
- Affordable switching strategies: Offering single enantiomers at cost levels comparable to racemates.
- Public-private partnerships: Encouraging optimization of existing drugs with partial public funding, reducing pressure for aggressive commercial recoupment.
- Transparent value communication: Clear public explanation of why a single enantiomer offers better value.
These models can help align corporate incentives with public good.
Conclusion
Chiral drugs illustrate the best and worst tensions in modern pharmaceutical innovation.
While science enables better, safer medicines, commercial forces sometimes distort incentives, challenging public trust.
Regulators, payers, companies, prescribers, and patients must work together to ensure that chirality-driven developments serve not only molecules but humanity.
In the next episode, we will explore the emerging scientific and regulatory challenges associated with complex chiral drugs -including those with multiple stereocenters and dynamic stereochemistry.
What is in the next episode?
“Beyond simple mirror images lie molecular mazes -join us for Episode 9: Challenges on the Horizon: Complex Chirality and Emerging Scientific Questions.“
References
Agranat, I., & Wainschtein, S. R. (2010). The strategy of enantiomer patents of drugs. Drug Discovery Today, 15(5-6), 163–170.
European Medicines Agency (EMA). (1994). Investigation of Chiral Active Substances (CPMP Note for Guidance 3CC29a).
Food and Drug Administration (FDA). (1992). Development of New Stereoisomeric Drugs (Policy Statement). Federal Register, 57(88), 22249–22250.
Hancu, G., & Modroiu, A. (2022). Chiral switch: Between therapeutical benefit and marketing strategy. Pharmaceuticals, 15(2), 240.