“When a drug transforms from a blend to a single hand, is it innovation -or a clever commercial sleight of hand?“
Introduction
The regulatory awakening around chiral drugs enabled not just better science but also a new strategic tool for the pharmaceutical industry: the chiral switch. This phenomenon -developing a single-enantiomer version of an already marketed racemate -became both a scientific opportunity and a commercial strategy. While some switches were genuine innovations that improved patient outcomes, others sparked ethical debates about evergreening, market exclusivity, and value to patients.
This episode explores the regulatory, scientific, ethical, and commercial dimensions of chiral switches, through notable case studies like esomeprazole, levocetirizine, and dexmethylphenidate.
What is a Chiral Switch?
Definition: A chiral switch refers to the development and marketing of a single-enantiomer version of an already approved racemic drug.
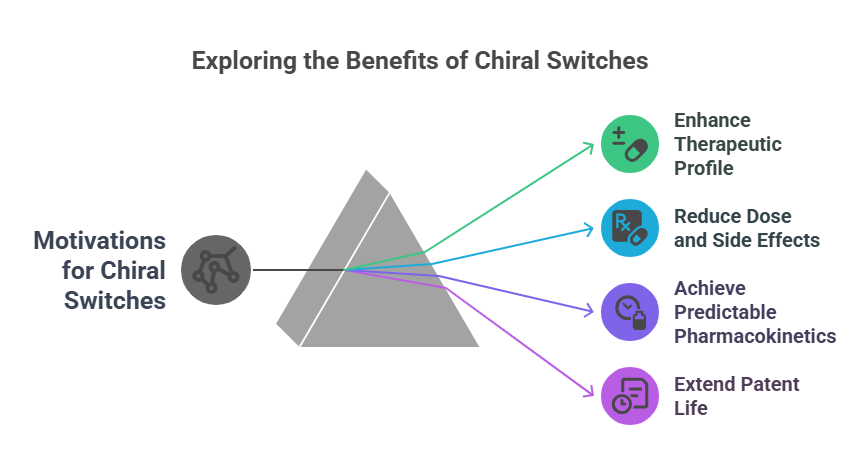
Motivations for Chiral Switches
- Enhance therapeutic profile (e.g., potency, safety).
- Reduce dose and side effects.
- Achieve more predictable pharmacokinetics.
- Extend patent life and commercial exclusivity (Agranat, Caner, & Caldwell, 2002).
Regulatory Expectations for Chiral Switches
Agencies like FDA, EMA, PMDA, and TGA have laid down clear expectations when it comes to chiral switches:
- New molecular entity (NME) status: A single-enantiomer product derived from a racemate is typically treated as an NME.
- Full NDA/MAA submission: Not a simple supplemental application -full nonclinical, clinical, and CMC packages are expected.
- Clinical superiority demonstration: Companies must demonstrate that the single enantiomer provides clinically meaningful advantages -not just chemical purity (FDA, 1992).
- Bioequivalence considerations: Comparative pharmacokinetic studies must establish how the single enantiomer differs from the racemate.
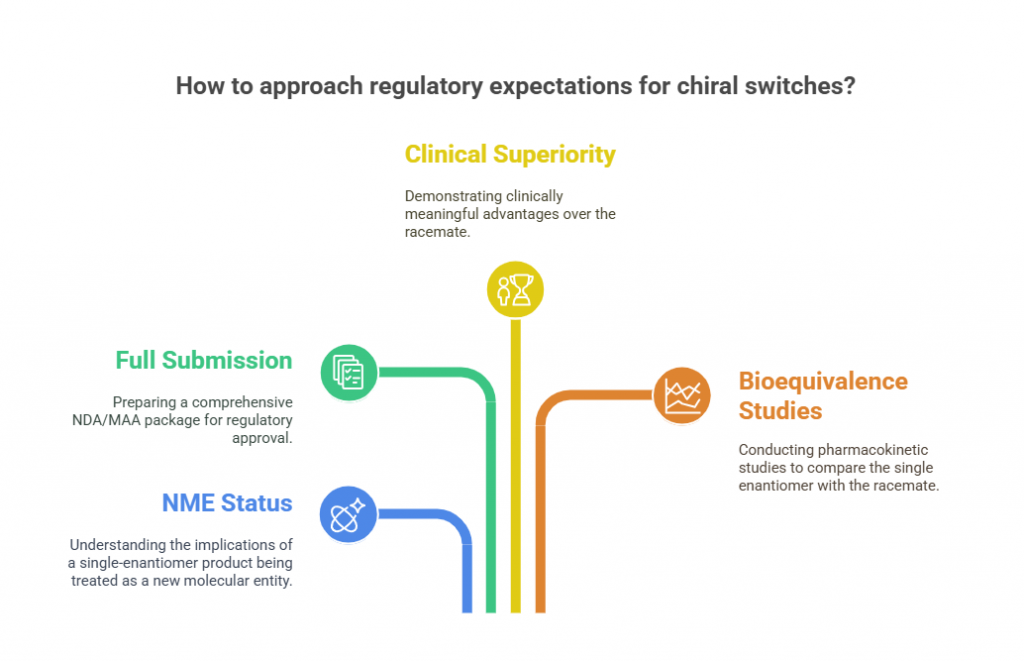
Failure to prove clear advantages can lead to regulatory challenges or market skepticism.
Scientific Justifications for Chiral Switches
Switches are scientifically justifiable if:
- Only one enantiomer is pharmacologically active.
- The other enantiomer contributes adverse effects.
- The racemate shows stereoselective metabolism leading to variable efficacy.
- Simplified dosing or better safety margins are possible.
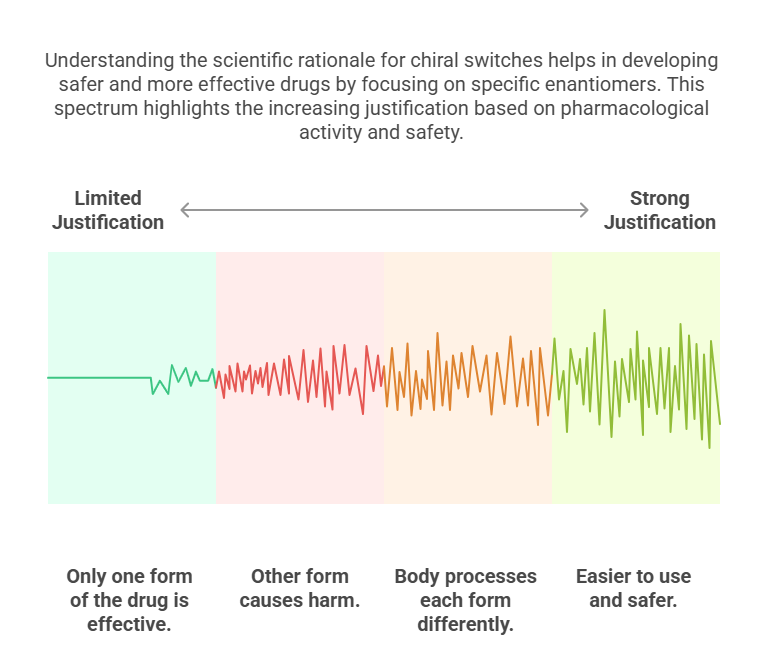
Without one or more of these justifications, regulators -and increasingly, payers and clinicians -question the rationale for the switch (Agranat & Wainschtein, 2010).
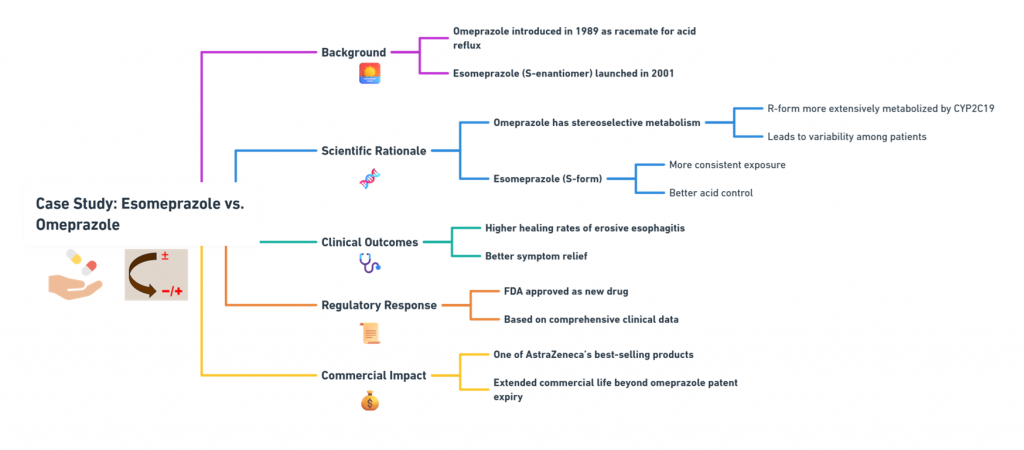
Case Study 1: Esomeprazole vs. Omeprazole
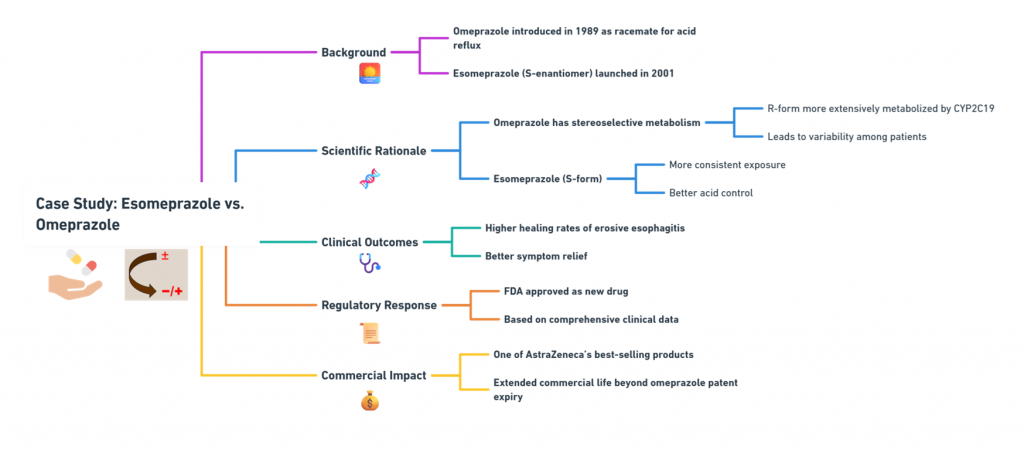
Background:
- Omeprazole was introduced in 1989 as a racemate for treating acid reflux.
- Esomeprazole, the S-enantiomer, was launched in 2001.
Scientific Rationale:
- Omeprazole exhibits stereoselective metabolism: the R-form is more extensively metabolized by CYP2C19, leading to variability.
- Esomeprazole (S-form) has more consistent exposure across patients, offering better acid control.
Clinical Outcomes:
- Studies demonstrated higher healing rates of erosive esophagitis and better symptom relief (Andersson, 2001).
Regulatory Response:
- Approved as a new drug by the FDA after comprehensive clinical data submission.
Commercial Impact:
- Esomeprazole became one of AstraZeneca’s best-selling products, extending the commercial life of the omeprazole franchise well beyond patent expiry.
Case Study 2: Levocetirizine vs. Cetirizine
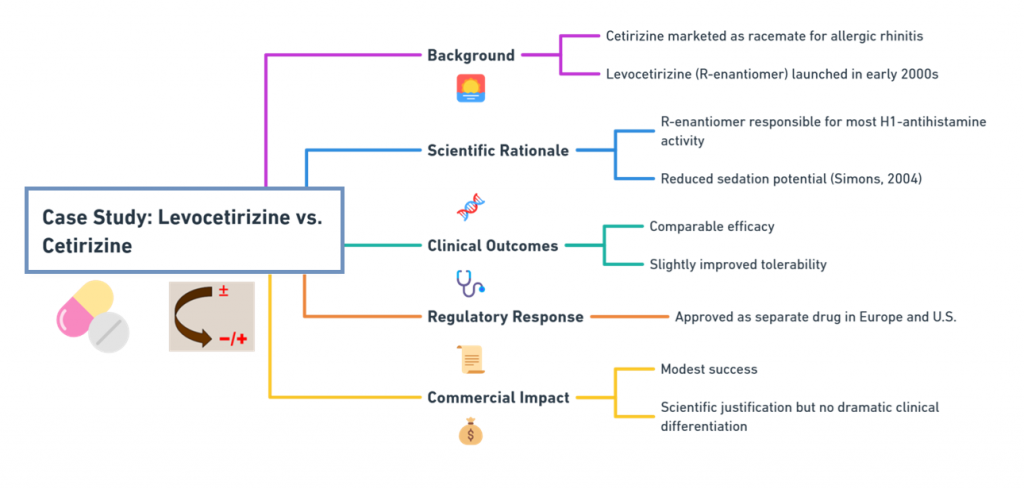
Background:
- Cetirizine was marketed as a racemate for allergic rhinitis.
- Levocetirizine, the R-enantiomer, was launched in the early 2000s.
Scientific Rationale:
- R-enantiomer responsible for most H1-antihistamine activity.
- Reduced sedation potential compared to the racemate (Simons, 2004).
Clinical Outcomes:
- Comparable efficacy but slightly improved tolerability.
Regulatory Response:
- Approved as a separate drug in Europe and the U.S.
Commercial Impact:
- Modest -although a scientific justification existed, the switch did not create dramatic clinical differentiation.
Case Study 3: Dexmethylphenidate vs. Racemic Methylphenidate
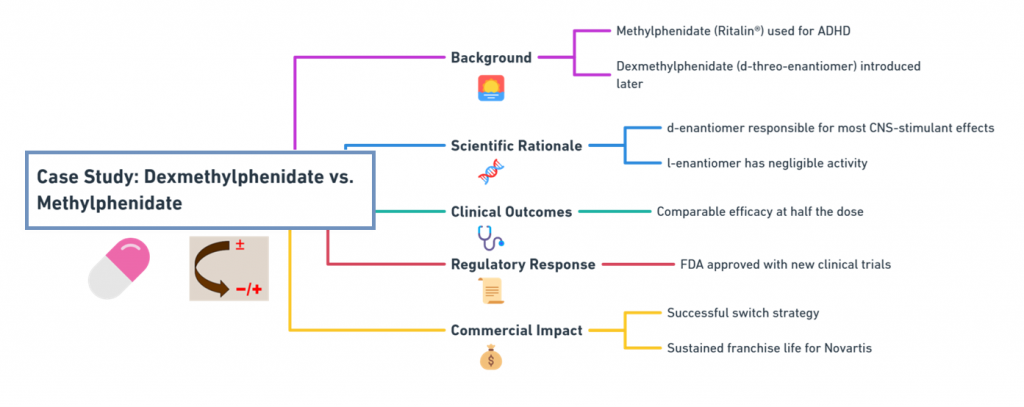
Background:
- Methylphenidate (Ritalin®) was used for ADHD.
- Dexmethylphenidate, the d-threo-enantiomer, was introduced later.
Scientific Rationale:
- The d-enantiomer is responsible for most CNS-stimulant effects.
- The l-enantiomer has negligible activity.
Clinical Outcomes:
- Comparable efficacy at half the dose.
Regulatory Response:
- Approved by the FDA with new clinical trials.
Commercial Impact:
- Successful switch strategy by Novartis to sustain franchise life.
Ethical Challenges of Chiral Switches
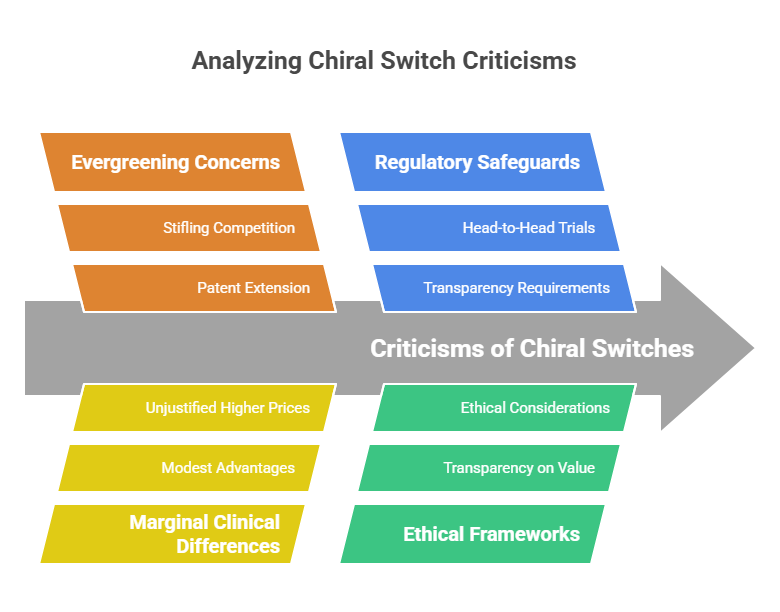
While some chiral switches represent legitimate therapeutic innovation, others have attracted criticism:
Evergreening Concerns
- Critics argue that some switches are used primarily to extend patent life and stifle generic competition without offering meaningful clinical improvement (Hancu & Modroiu, 2022).
Marginal Clinical Differences
- Some single-enantiomer versions show only modest advantages, insufficient to justify higher prices.
Regulatory Safeguards
- Agencies increasingly require head-to-head clinical trials against the racemate to demonstrate meaningful benefit.
- Ethical frameworks now push for transparency on the true value of a switch (EMA, 1994).
Payer and Prescriber Perspectives
- Insurance payers often require proof of significant therapeutic advantage before covering a more expensive single-enantiomer version.
- Prescribers are cautious about switching patients from a well-tolerated racemate to a new enantiomer without clear reasons.
- Patients are increasingly aware of chiral switch controversies, influencing public trust.
Thus, even if regulators approve a chiral switch, market adoption is not guaranteed unless the clinical value proposition is clear.
Regulatory Trends: Tougher Standards for Switches
Modern regulatory expectations are more stringent:
- FDA and EMA now expect not only PK improvements but clear clinical benefit.
- Comparative efficacy studies against the racemate are often needed.
- Labeling must clearly explain differences in pharmacology, efficacy, and safety.
Simply achieving better chiral purity without superior outcomes no longer suffices.
Future of Chiral Switches
Looking forward:
- True chiral switches based on genuine clinical benefit will continue to be pursued.
- Regulatory scrutiny will ensure only scientifically justified switches succeed.
- Innovations in asymmetric synthesis and chiral drug design will favor initial single-enantiomer development, reducing the need for later switches.
In short: the bar for chiral switches is higher than ever -and rightly so.
Conclusion
Chiral switches exemplify both the opportunities and challenges at the intersection of science, regulation, and commerce.
When grounded in genuine clinical benefit, they enhance therapy and serve public health.
When driven solely by commercial motivations, they risk undermining trust and value.
Regulators today navigate this complex landscape with increasing vigilance, ensuring that when a drug switches from two hands to one, it does so for the right reasons.
In the next episode, we will explore how regulators and scientists evaluate whether single enantiomers are truly superior -clinically, ethically, and economically.
What is in the next episode?
“Is the better twin always truly better? Find out in Episode 7: Clinical Questions: Are Single Enantiomers Always Better?.“
References
Agranat, I., Caner, H., & Caldwell, J. (2002). Putting chirality to work: The strategy of chiral switches. Nature Reviews Drug Discovery, 1(10), 753–768.
Agranat, I., & Wainschtein, S. R. (2010). The strategy of enantiomer patents of drugs. Drug Discovery Today, 15(5-6), 163–170.
Andersson, T. (2001). Pharmacokinetics, metabolism and interactions of acid pump inhibitors: Focus on omeprazole, lansoprazole, pantoprazole, and rabeprazole. Clinical Pharmacokinetics, 40(7), 523–538.
European Medicines Agency (EMA). (1994). Investigation of Chiral Active Substances (CPMP Note for Guidance 3CC29a).
Food and Drug Administration (FDA). (1992). Development of New Stereoisomeric Drugs (Policy Statement). Federal Register, 57(88), 22249-22250.
Hancu, G., & Modroiu, A. (2022). Chiral switch: Between therapeutical benefit and marketing strategy. Pharmaceuticals, 15(2), 240.
Simons, F. E. (2004). Advances in H1-antihistamines. New England Journal of Medicine, 351(21), 2203–2217.
Further Reading