Tagline:
“When two mirror images offer different paths, how do regulators choose which one should become medicine?”
Introduction
The rise of regulatory consciousness around chiral drugs in the 1990s opened a crucial new question:
If mirror-image molecules behave differently, how should the pharmaceutical industry -and regulators -decide which enantiomer to develop, approve, and market?
This episode explores how regulatory agencies like the FDA, EMA, PMDA, CDSCO, and TGA crafted expectations for the development, approval, and control of single-enantiomer drugs. It covers the scientific justifications regulators demand, the critical technical requirements for purity and stability, and the ongoing clinical and ethical debates about when a single-enantiomer strategy is justified.
In a world where left and right-handed molecules can change lives, choosing the right “twin” has become a matter of public trust, not just chemistry.
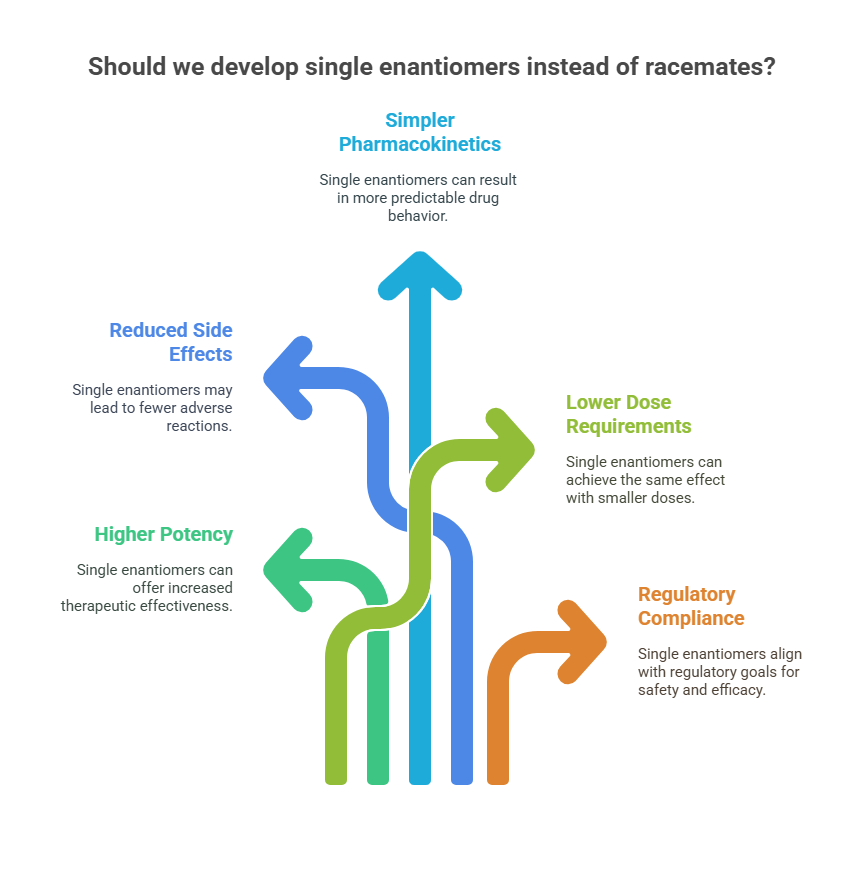
Why Develop Single Enantiomers?
Initially, racemates were the norm because they were easier and cheaper to produce. However, the increasing ability to develop single enantiomers raised new possibilities:
- Higher therapeutic potency (e.g., esomeprazole vs. omeprazole).
- Reduced side effects (e.g., levocetirizine vs. cetirizine).
- Simpler pharmacokinetics with less variability.
- Lower dose requirements leading to cost savings or better patient adherence.
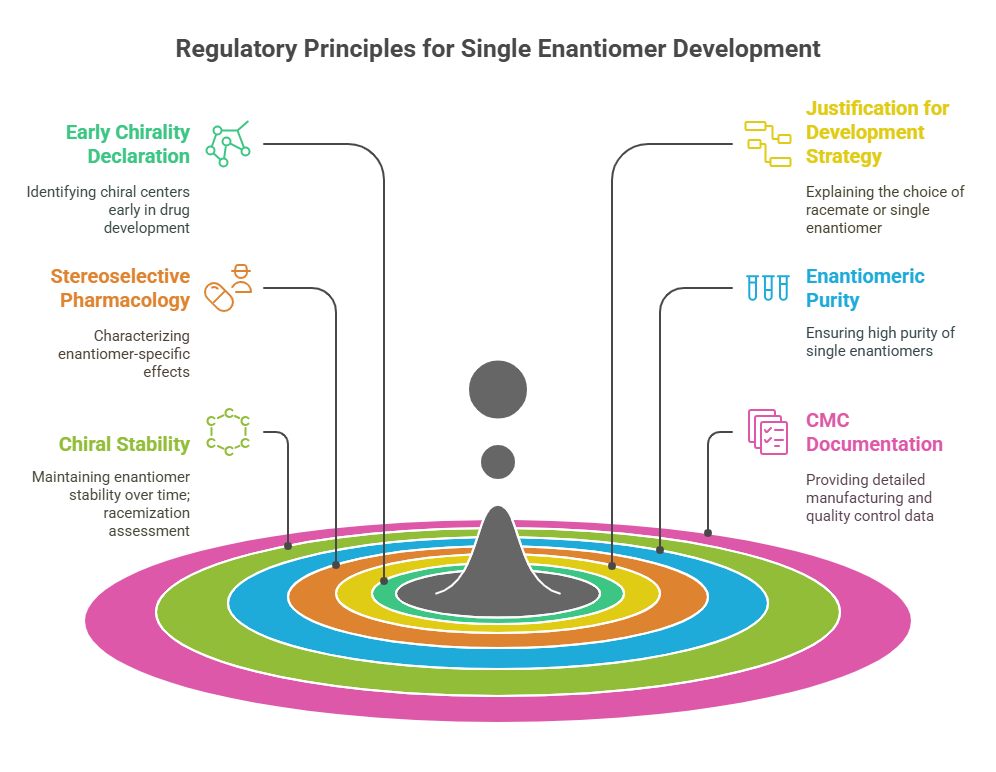
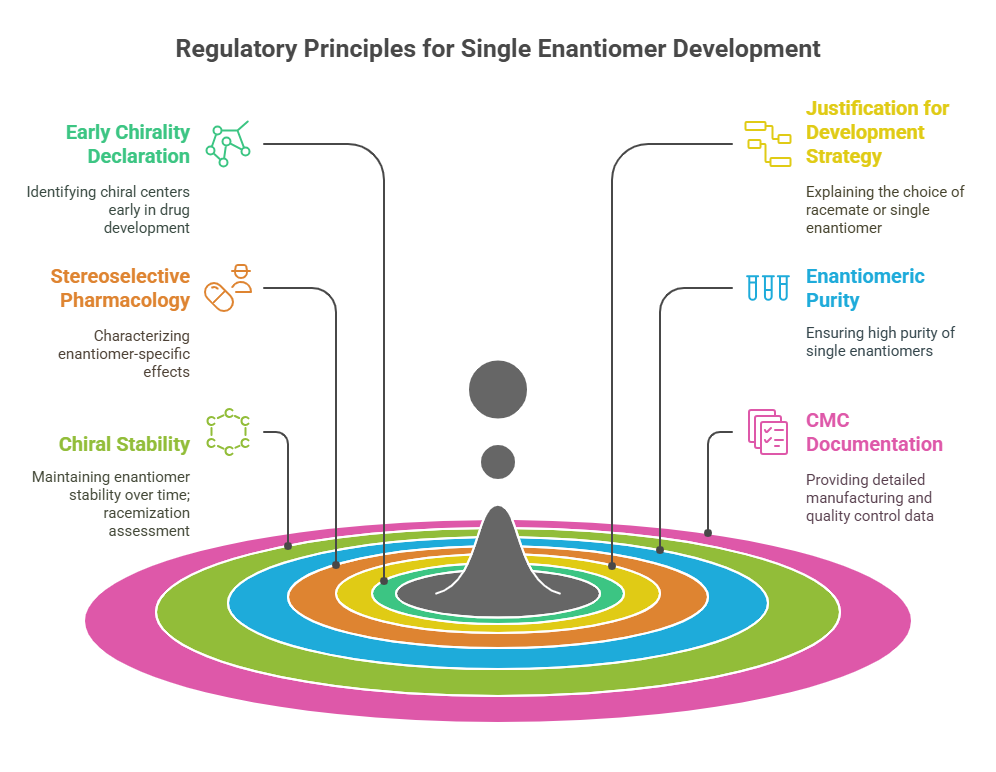
Regulatory Principles for Single Enantiomer Development
Across major agencies, several core expectations guide single-enantiomer drug development:
1. Early Declaration of Chirality
Drug developers are expected to identify the presence of chiral centers as early as possible -typically during lead optimization or preclinical development.
- FDA: Requires early characterization of stereochemistry at the Investigational New Drug (IND) stage (FDA, 1992).
- EMA: Insists chirality be acknowledged at the beginning of the dossier (EMA, 1994).
- PMDA and TGA: Follow similar expectations, often referencing ICH Q6A for quality documentation (ICH, 1999).
This early identification ensures that stereochemistry is not treated as an afterthought.
2. Justification for Development Strategy
When a molecule is chiral, developers must justify whether they intend to market:
- A racemate,
- A purified single enantiomer,
- Or both forms sequentially (e.g., a racemate followed by a chiral switch).
Scientific factors considered include:
- Differential pharmacodynamics and pharmacokinetics between enantiomers.
- Toxicology profiles.
- Stability (i.e., propensity for racemization).
- Technical feasibility and manufacturing cost.
If the enantiomers show marked differences in efficacy, toxicity, or metabolism, regulators expect a strong justification for not pursuing a single-enantiomer drug (Agranat et al., 2002).
3. Stereoselective Pharmacology and Toxicology (Chiral Pharmacology)
A central expectation is that sponsors must characterize the pharmacological and toxicological profiles of each enantiomer separately, unless justified otherwise.
For example:
- Binding affinity of each enantiomer to therapeutic and off-target receptors.
- Functional activity assays (e.g., agonism vs. antagonism).
- Stereoselective toxicity (e.g., one enantiomer causing cardiotoxicity while the other is benign).
FDA’s 1992 guidance stresses the need for separate evaluation whenever the enantiomers can be individually isolated and studied (FDA, 1992).
4. Enantiomeric Purity Requirements
When a single-enantiomer drug is developed, regulators impose strict requirements for chiral purity:
- Typically ≥98% enantiomeric excess (ee) is expected.
- The undesired enantiomer is treated similarly to an impurity (distomer) and must be quantified and controlled (ICH, 1999).
- Analytical methods must be validated for enantiomeric specificity, typically using chiral HPLC or SFC (Lesellier, 2008).
This ensures that batch-to-batch variability does not unintentionally affect pharmacological or safety profiles.
5. Chiral Stability: Racemization Assessment
If a single enantiomer is selected, developers must demonstrate that:
- It remains stable during manufacturing, storage, and administration.
- It does not undergo significant in vivo racemization after administration (Wainer, 1993).
For example:
- Thalidomide, with a labile chiral center, undergoes rapid in vivo racemization, making single-enantiomer development ineffective for eliminating teratogenic risks (McBride, 1961).
Thus, stability studies must include:
- Forced degradation experiments.
- Long-term and accelerated stability studies.
- In vivo pharmacokinetic investigations.
6. CMC (Chemistry, Manufacturing, and Controls) Documentation
Quality modules of regulatory submissions must address:
- Manufacturing routes designed for asymmetric synthesis or chiral separation.
- Specifications for chiral purity at the drug substance and drug product stages.
- Validation of chiral analytical methods under GMP conditions.
Without robust CMC control, approval of a single-enantiomer drug may be delayed or denied.
Case Study: Esomeprazole
Esomeprazole (the S-enantiomer of omeprazole) offers a compelling example of how regulators applied these expectations.
- Pharmacodynamics: Similar potency to omeprazole.
- Pharmacokinetics: Reduced interindividual variability compared to racemic omeprazole.
- Clinical Impact: More consistent acid suppression and better healing rates in GERD patients (Andersson, 2001).
- Regulatory outcome: Approved as a new molecular entity (NME) by the FDA after demonstration of clinical advantage.
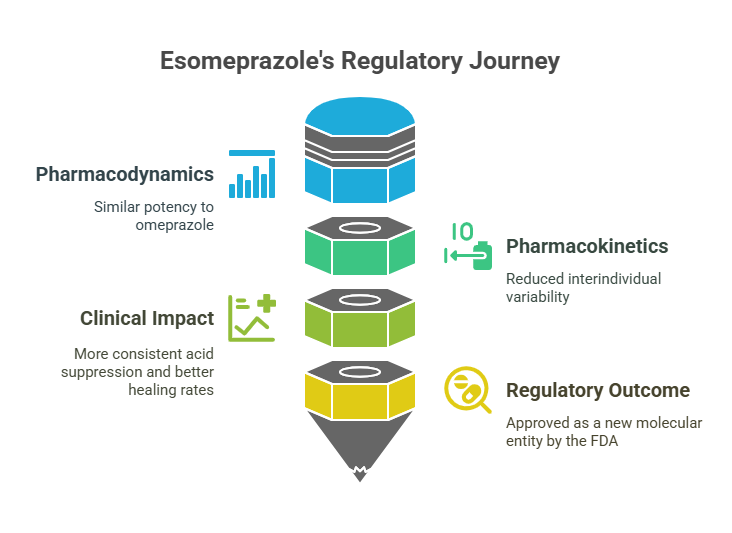
This success illustrates that single-enantiomer development is not just a regulatory checkbox -it must deliver real-world clinical benefit to be justified.
Chiral Switches: Special Regulatory Considerations
When companies develop a single-enantiomer version of an already marketed racemate -a “chiral switch” – regulators impose additional scrutiny:
Requirements typically include:
- Proof that the single enantiomer is not simply a “me-too” version without significant improvement.
- New clinical data demonstrating therapeutic advantages.
- Intellectual property concerns must not drive development absent clinical merit (Agranat & Wainschtein, 2010).
Some switches have been accepted (e.g., levocetirizine) based on improved side effect profiles, while others have been viewed skeptically.
Ethical and Practical Challenges
Even with clear regulatory expectations, challenges persist:
- Marginal advantages: Some single-enantiomer drugs offer only slight clinical improvements, raising ethical questions about pricing and marketing (Hancu & Modroiu, 2022).
- Patient confusion: Switching from racemate to enantiomer formulations can create adherence challenges if not clearly explained.
- Patent extensions: Critics argue that some chiral switches are driven more by commercial lifecycle management than patient benefit.
Regulators thus face a delicate balance between encouraging scientific innovation and protecting against strategic manipulation.
Conclusion
The development of single-enantiomer drugs represents a confluence of scientific, regulatory, and ethical considerations. Regulatory agencies today expect -and enforce -a sophisticated, evidence-based approach to stereochemistry.
Choosing the “right twin” is no longer a matter of manufacturing convenience or commercial strategy. It is a carefully engineered process requiring proof of advantage, control of purity, assurance of stability, and clarity of clinical benefit.
In the next episode, we will explore the phenomenon of chiral switches more deeply, examining how regulators evaluate whether a switch represents true therapeutic innovation -or simply commercial opportunism.
What is in the next episode?
“When a racemate becomes two drugs, what’s gained and what’s lost? Join us in Episode 6: The Rise of the Chiral Switch: Strategy or Science?.“
References
Israel Agranat & Ilaria D’Acquarica (2025), Racemic drugs are not necessarily less efficacious and less safe than their single-enantiomer components. European Journal of Pharmaceutical Sciences. https://doi.org/10.1016/j.ejps.2025.107082
Hancu, G., & Modroiu, A. (2022). Chiral switch: Between therapeutical benefit and marketing strategy. Pharmaceuticals, 15(2), 240.
Agranat, I., Caner, H., & Caldwell, J. (2002). Putting chirality to work: The strategy of chiral switches. Nature Reviews Drug Discovery, 1(10), 753–768.
Agranat, I., & Wainschtein, S. R. (2010). The strategy of enantiomer patents of drugs. Drug Discovery Today, 15(5-6), 163–170.
Andersson, T. (2001). Pharmacokinetics, metabolism and interactions of acid pump inhibitors: Focus on omeprazole, lansoprazole, pantoprazole, and rabeprazole. Clinical Pharmacokinetics, 40(7), 523–538.
European Medicines Agency (EMA). (1994). Investigation of Chiral Active Substances (CPMP Note for Guidance 3CC29a).
Food and Drug Administration (FDA). (1992). Development of New Stereoisomeric Drugs (Policy Statement). Federal Register, 57(88), 22249-22250.
International Council for Harmonisation (ICH). (1999). Q6A: Specifications – Test Procedures and Acceptance Criteria for New Drug Substances and New Drug Products: Chemical Substances.
Lesellier, E. (2008). The many faces of packed column supercritical fluid chromatography -A critical review. Journal of Chromatography A, 1217(11), 1560–1580.
McBride, W. G. (1961). Thalidomide and congenital abnormalities. The Lancet, 278(7216), 1358.
Wainer, I. W. (1993). The regulatory requirements for the separation, identification, quantitation, and pharmacological evaluation of the enantiomers of chiral drugs. Journal of Pharmaceutical and Biomedical Analysis, 11(6), 519–526.
Further Reading