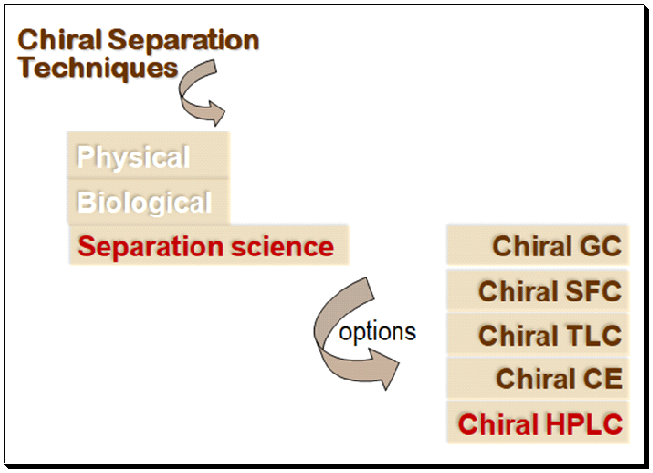
What is chiral chromatography?
Chiral chromatography is now widely understood and applied in daily life. The correct phrase, however, is “enantioselective chromatography”. Chiral chromatography has developed into the most popular method for enantiomeric purity assessment and pure enantiomer separation, both on an analytical and preparative scale. The initial step in any investigation into enantioselective synthesis or separation is a chiral chromatographic assay. This includes employing strategies like High performance liquid chromatography (HPLC), chiral supercritical fluid chromatography (SFC), capillary electrophoresis (CE), thin-layer chromatography (TLC), gas chromatography (GC), and thin-layer chromatography (TLC). The results of a literature review show that HPLC-based chiral assays are the most widely used technology.
A summary of the various analytical methods engaged for chiral separation and analysis are shown below.
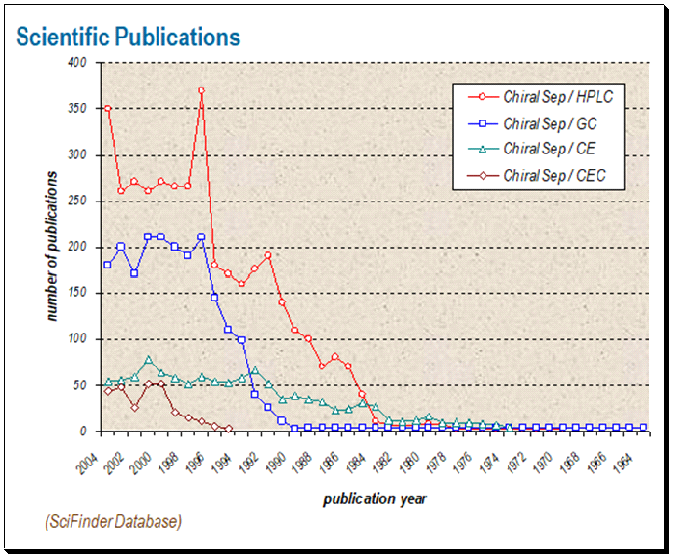
The result of a literature survey done identifies HPLC-based chiral assays as the most dominating technology in use. One can find that chiral HPLC technique is at the top with approximately 375 publications. This is followed by chiral GC, chiral CE and last separation by chiral CEC.
Principle and application of chiral chromatographic techniques – a brief narrative
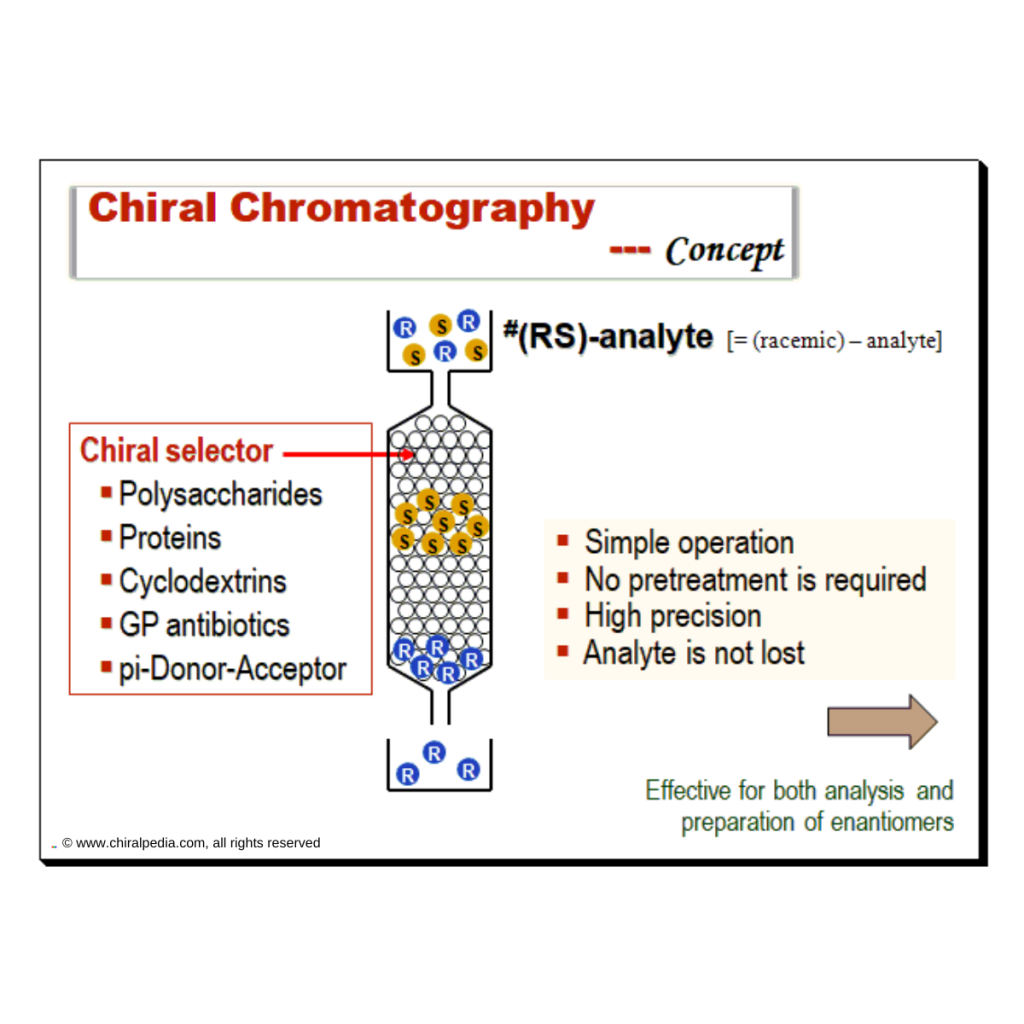
A brief description of the principle and application capabilities of commonly employed chiral chromatographic separation techniques is presented in the table below.
| Method | Brief narrative of principle and application |
|---|---|
| Chiral HPLC | Chiral HPLC is used to separate enantiomers either by direct or indirect separation mode. Widely employed to assess chiral purity, provided the reference standards of the racemate or the two enantiomers are available. Capable of distinguishing between enantiomers and from the racemate; (+) from (-) and (±) |
| Chiral GC | Majority of chiral separations using GC are done with cyclodextrin derivatives as chiral selector. This method can be employed to distinguish between enantiomers and from the racemate; (+) from (-) and (±) |
| Supercritical fluid chromatography (SFC) | Principle is very similar to that of HPLC. But SFC usually uses carbon dioxide as the mobile phase. Hence there is a need to pressurize the entire chromatographic flow path. SFC can differentiate enantiomers and enantiomer from the racemate; (+) from (-) and (±) |
| Chiral capillary electrophoresis (CE) | Chiral CE is based mostly on separation of enantiomers by complex formation with cyclodextrins which is used as the chiral selector. Able to differentiate between enantiomers and from the racemate; (+) from (-) and (±) |
Need for chiral chromatography
Numerous substances of biological and pharmaceutical value are chiral. Pharmacodynamic, pharmacokinetic, and toxicological properties of the enantiomers of racemic chiral drugs have grown significantly and have become a major concern for the pharmaceutical industry and regulatory organizations. Typically, one of the enantiomers is more pharmacologically active (eutomer). The inactive enantiomer (distomer) may occasionally cause undesired side effects or even hazardous effects. Even if the negative effects are not severe, the inactive enantiomer must be metabolized, placing an extra pressure on the patient’s already overworked system. The necessity for correct assessment of the enantiomeric purity of pharmaceutical, agrochemical, and other chemical entities including fragrances and flavors becomes critical when there are significant changes in activity between enantiomers. Additionally, the enantioselective ADME process means that a racemic drug is no longer 50:50 the instant it is introduced into a biological system, a chiral environment. As a consequence there is a need for chiral chromatographic technique for chiral separation, analysis, and monitoring of mirror-image molecules in the chiral drug discovery and development process.
Challenges in chiral chromatography (enantiomer separation)
In an isotopic/achiral environment, enantiomers exhibit identical physicochemical properties, and therefore are indistinguishable under these conditions. The separation of chiral molecules poses a unique challenge. To achieve separation of enantiomeric molecules on need to construct the right chiral environment. To drive this point home let us analyze the assay of ibuprofen, a NSAID drug, as an illustrative example. Ibuprofen has a single stereogenic center and exists as a pair of mirror-image molecules. It carries a carboxylic function.
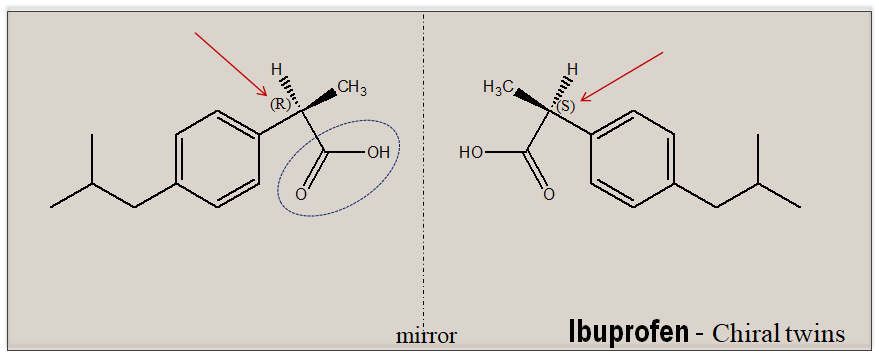
In a routine laboratory exercise ibuprofen is estimated by an alkalimetric titration exploiting the carboxylic function present in the molecule. This will provide only the total content of ibuprofen but will not tell you about how much each of the enantiomer is present since the estimation is done in an achiral environment (an achiral environment cannot differentiate left handed from the right handed molecules). To separate and quantify how much each of the enantiomer ((R)-and (S)-ibuprofen is present one need to perform the experiment in a chiral environment, where the differences between enantiomeric pair are more pronounced and become distinguishable. Hence, for chiral examination there is a need to have the right chiral environment. This could be provided as a plane polarized light, an additional chiral compound or by exploiting the inborn chirality of nature.
Further reading
- Beesley, Thomas E; P.W. Scott, Raymond (2001-05-30). John Wiley & Sons, Ltd (ed.). Chiral chromatography. USA: Wiley. doi:10.1002/047001590x. ISBN 978-0-470-01617-6.
- Wozniak, Timothy J.; Bopp, Ronald J.; Jensen, Eric C. (1991). “Chiral drugs: An industrial analytical perspective”. Journal of Pharmaceutical and Biomedical Analysis. 9 (5): 363–382. doi:10.1016/0731-7085(91)80160-b. ISSN 0731-7085. PMID 1932271.
- Francotte, Eric; Lindner, Wolfgang (2006). Chirality in drug research. Eric Francotte, W. Lindner. Weinheim: Wiley-VCH. ISBN 978-3-527-60943-7. OCLC 163578005.
4. Chiral Analysis. Elsevier. 2018. doi:10.1016/c2017-0-00050-2. ISBN 978-0-444-64027-7.
5. Wozniak, Timothy J.; Bopp, Ronald J.; Jensen, Eric C. (1991). “Chiral drugs: An industrial analytical perspective”. Journal of Pharmaceutical and Biomedical Analysis. 9 (5): 363–382. doi:10.1016/0731-7085(91)80160-b. ISSN 0731-7085. PMID 1932271.
6. Ahuja, Satinder (2011). Chiral separation methods for pharmaceutical and biotechnology products. New Jersey: John Wiley & Sons, Inc., New Jersey. ISBN 978-0-470-40691-5.
7. Chiral analysis. Wikipedia, Wikipedia Foundation, 27/10/2022. https://en.wikipedia.org/wiki/Chiral_analysis

Good article. Please do take seminar for chiral chromatography.
Dear Prof.,
Thank you for the Chiral Chromatography series. This is a much needed and underserved topic in the curriculum. The recent advances in chirality, as well as the Nobel Prize in Chemistry, demonstrate the relevance of this field.
I’m looking forward to this series.
regards,
Dear Dr. Chandramouli,
Thanks for your observation, interest and support.
Regards,