Atropisomers are stereoisomers resulting from hindered rotation about single bonds where the steric strain barrier to rotation is high enough to allow for the separation of the conformers. Note: Butane, for example, has conformations that are atropisomers; however, unlike the biaryls, the barrier to rotation is so small that they are interconverted rapidly at room temperature, and they are, for practical purposes, achiral.
As a general rule of thumb, chiral molecules must have at least one stereogenic center – that is, a carbon that has four unique substituents coming off it. However, like most rules of thumb, there are exceptions, and there are indeed examples of chiral molecules that have no chiral centers, a few classes of which are mentioned below. Molecules that do not possess a stereogenic center as a part of their structure may also exist in enantiomeric forms as a result of an axis or plane of chirality. Such structures occur less frequently in compounds of pharmaceutical interest. Among the several molecular assemblies able to display axial chirality, well known examples include biaryls, allenes, and spiranes. This post focuses on axial chirality in biphenyl systems and allenes.
The Biaryls
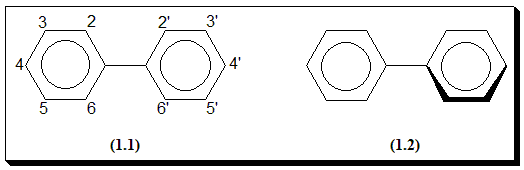
In the molecule of biphenyl there exists a possibility of rotation about the single bond linking the two-phenyl groups so that biphenyls could conceivably exist in a form (1.1) in which the two rings are coplanar, in a form (1.2) in which the two rings are perpendicular to each other, and in any forms between these two extremes. In the coplanar form the overlap of p orbital of the two rings allows maximum resonance stabilization but there is some inhibiting steric interaction of the o-hydrogen atoms (2 and 2’, 6 and 6’). In the perpendicular from resonance stabilization is at minimum but also is steric interaction. Electron diffraction studies suggest that the two rings are normally inclines at about 40°.
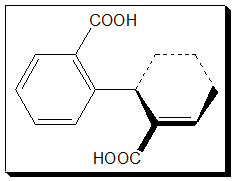
The steric interactions become more significant as the o-hydrogen atoms are replaced by bulkier groups and in diphenic acid (1.3), for example, the angle between the two rings is about 60°.
In a suitable substituted biphenyl compound the non-planar form exists in two enantiomeric structures. In summary, there are two conditions for enantiomerism in biphenyl (1.4)
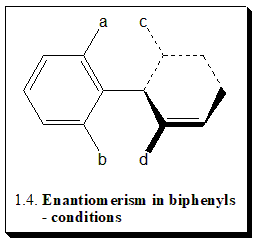
- The molecule must not contain a plane of symmetry; this requirement is met either if a ≠ b and c ≠ d or if a ≠ b and c = d with a m-substituent in this ring
- There must be sufficiently large barrier between the enantiomeric forms to prevent rapid interconversion.
Most enantiomeric biphenyls have three or four substituents in the o-positions to provide necessary optical stability.
This is illustrated by the three biphenyl derivatives (1.5 A, B, and C). In each of these compounds rotation is restricted as a result of the presence of bulky ortho groups. In the first case (1.5 A): the molecule has two planes of symmetry. If a plane is drawn containing all atoms and groups in either ring, it is a symmetrical perpendicular bisector of the other ring. Such molecules are not chiral.
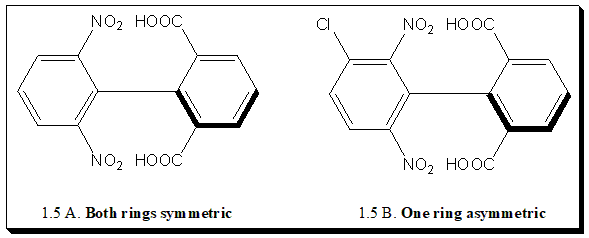
In the second case (1.5 B): only one ring is symmetric. A plane drawn in the ring containing the nitro groups symmetrically bisects the other ring, but the plane of the ring containing the carboxyl groups is not a plane of symmetry. Nevertheless, one plane of symmetry is sufficient to make the compound achiral, and hence molecule (B) is achiral.
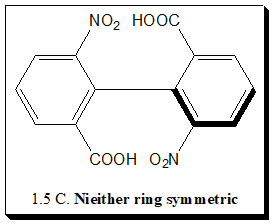
In the third case (1.5 C): neither ring is symmetric. There is no plane of symmetry, and the molecule is chiral. In other words two separate chemical species exist. Many such compounds have been separated. It is not always necessary for four large ortho groups to be present in order for rotation to be prevented. Compound with three or even two groups, if large enough can offer hindrance to rotation and, if suitably substituted, can be resolved.
Live examples
The experimental antifertility and antiviral agent gossypol has axis of chirality and hence can exist in two enantiomeric forms. Methaqualone, the anxiolytic and hypnotic-sedative, antibiotic vancomycin and antiulcer agent telenzepine also exhibit this phenomenon, atropisomersim.
Naming Atropisomers
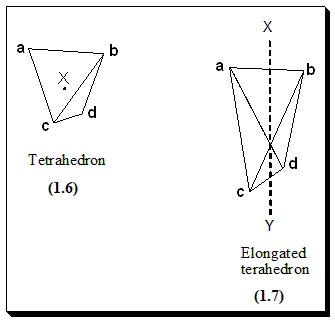
The specification of absolute configuration of biphenyl compounds is carried out as follows. Since biphenyls do not owe their chirality to the presence of chiral center, the criterion now is the presence of chiral axis. This chiral axis may be derived from a chiral center X (1.6), by ‘extending’ the point into a line XY that now passes through an elongated tetrahedron, (1.7). For X to be a chiral center (1.6), a, b, c and d all must be different, but for XY to be chiral axis (1.7), it is sufficient that a ≠ b and c ≠ d; To apply sequence the rule to axial chirality, it is necessary to use an additional rule; groups at the near end of the axis are given precedence over groups at the far end.
If (1.7), is viewed from point X, then the pair a-b, being nearer X, precedes the pair c-d; and vice-versa. As a result, viewing the molecule either from X or Y end is equivalent. If the order of priority is a>b>c>d, viewing the molecule keeping the low priority group (d) away from the observer, the molecule (1.7) gets the (R)-configuration.
Consider the following substituted biphenyl (1.8) to illustrate the concept
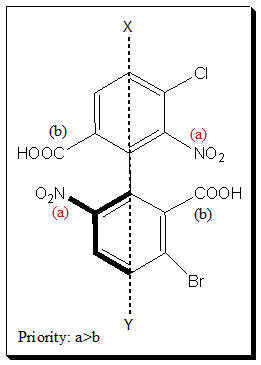
First the four ortho-groups are inspected. In the above structure, NO2 = a, and COOH = b (priority a>b), and according to the sequence rule this molecule is assigned S-configuration. [Construct molecular model and practice-assigning configuration]
The Allenes
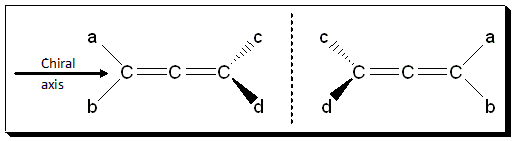
Allenes (1.9), for example, compounds containing cumulated double bonds, can be separated into enantiomers, even though there are no chiral centers. The central carbon in an allene is sp hybridized and the substituents at either end are orthogonal to one another, with one side of substituents going up and down in the vertical plane, and the other side coming into and out of the horizontal plane. Because of the rigidity of the double bonds, free rotation cannot occur at normal temperatures and pressures, and there is no interconversion between the two enantiomers, and they can be separated from each other. For the allene is exhibit chirality it should comply with the condition that a ≠ b and c ≠ d.
Live examples
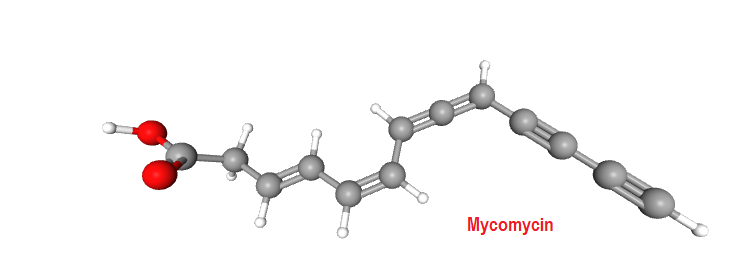
There are a few examples of drug molecule having an allene system in their structure. The natural antibiotic mycomycin, an antibiotic with tuberculostatic properties and nemotinic acid, are typical examples that exhibit atropisomersim.
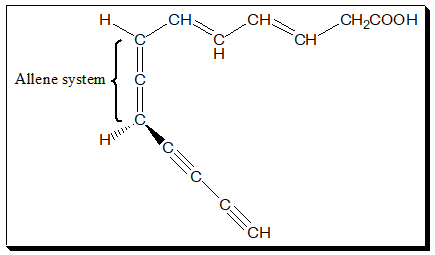
Practice exercise
Try to assign absolute configuration to Mycomycin. Structure is given above
References
Jerry March, Advanced Organic Chemistry: Reactions, Mechanisms and Structure, John Wiley & Sons Inc., New York, 2006.
Applying R,S descriptors to biaryls. 2013; https://personalpages.manchester.ac.uk/staff/T.Wallace/20412tw2/3.2.4_RS_in_biaryls.html
https://www.science.org/content/blog-post/two-molecules-when-you-were-expecting-just-one
Atropisomers. Wikipedia, Wikipedia Foundation, 10/03/2022. https://en.wikipedia.org/wiki/Atropisomer
Bernard Testa, Principles of organic stereochemistry, Marcel Dekker Inc., New York, 1979.

It is very useful for us much appreciated
Simple and clear. Live examples are really a additional learning . Thank you sir.
Thanks for sharing your observation. Glad you liked the blog on ‘Atropisomers’.