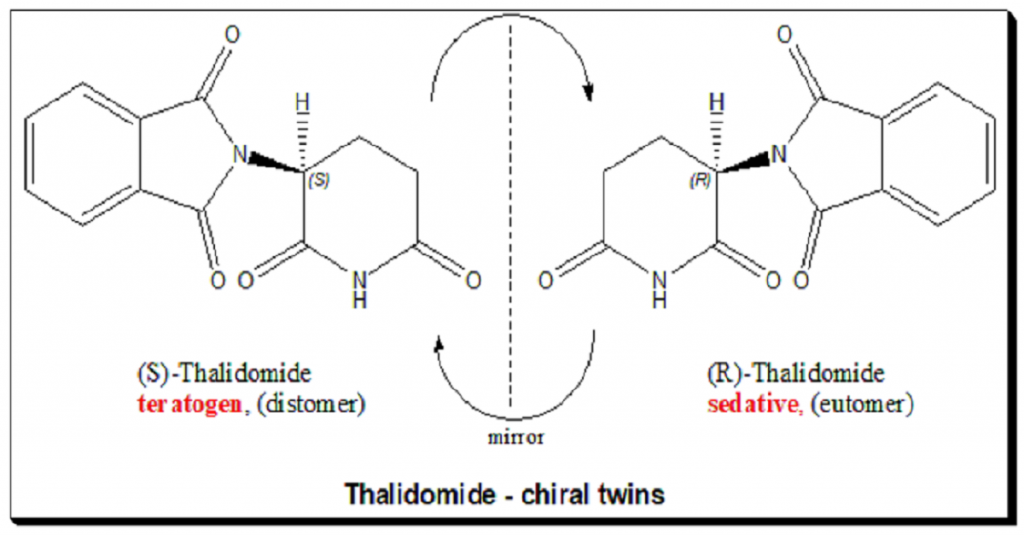
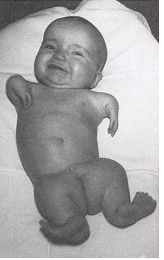
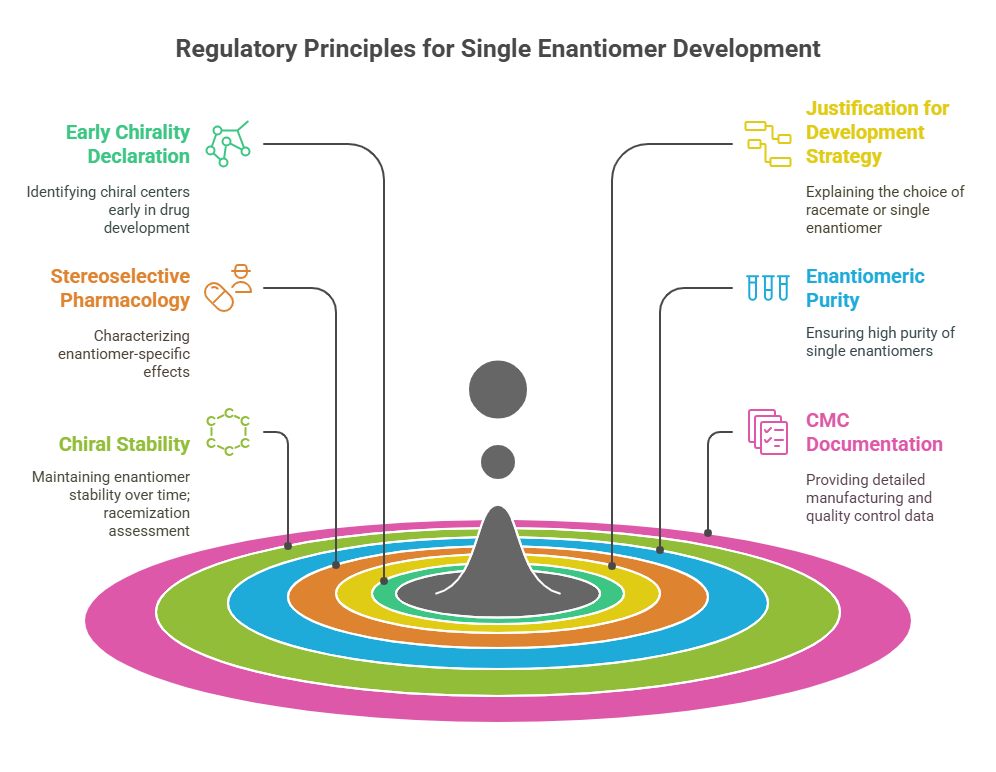
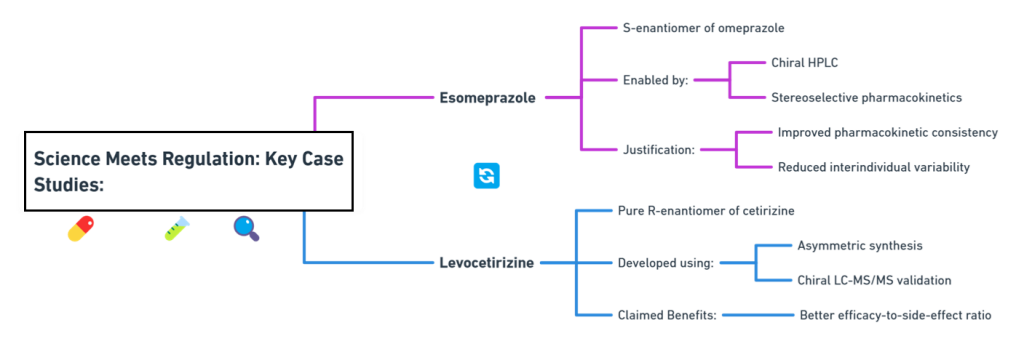
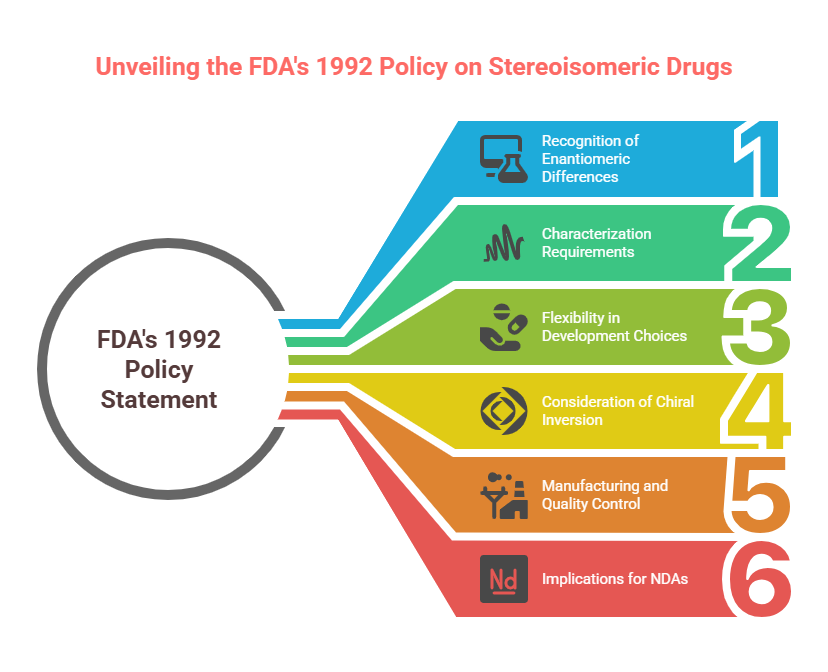
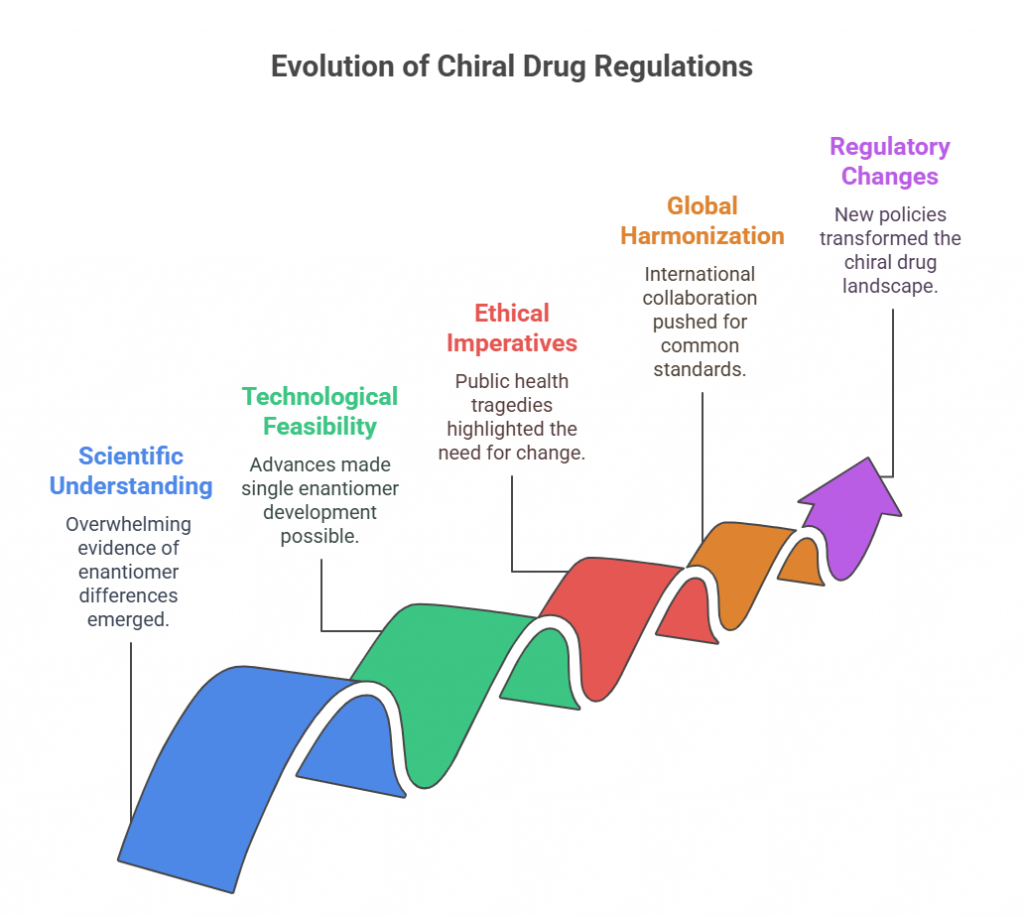
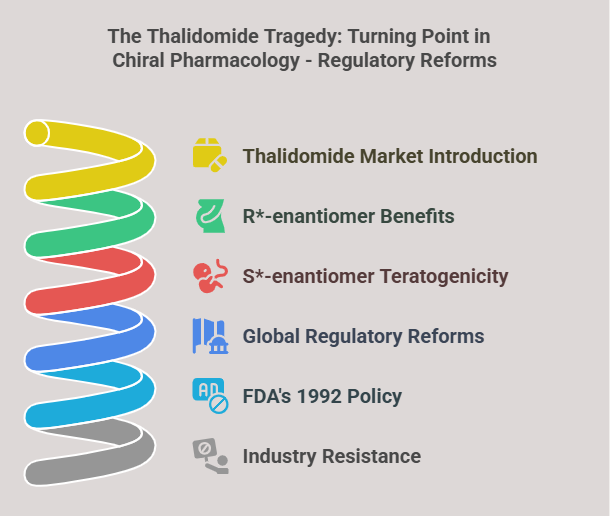
Thalidomide
Thalidomide was a racemic therapeutic and prescribed to pregnant women to control nausea and vomiting. The drug was withdrawn from world market when it became evident that the use in pregnancy causes phocomelia (clinical conditions where babies are born with deformed hand and limbs). Later in late 1970s, the thalidomide enantiomers were separated and enantioselective studies indicated that the (R)- enantiomer, the good partner, is an effective sedative, the (S)-enantiomer, the evil partner, harbors teratogenic …