Where a molecule’s handedness meets clever engineering—shaping safer, more precise, and more effective medicines.
Introduction: Why Chirality Matters in Drug Engineering
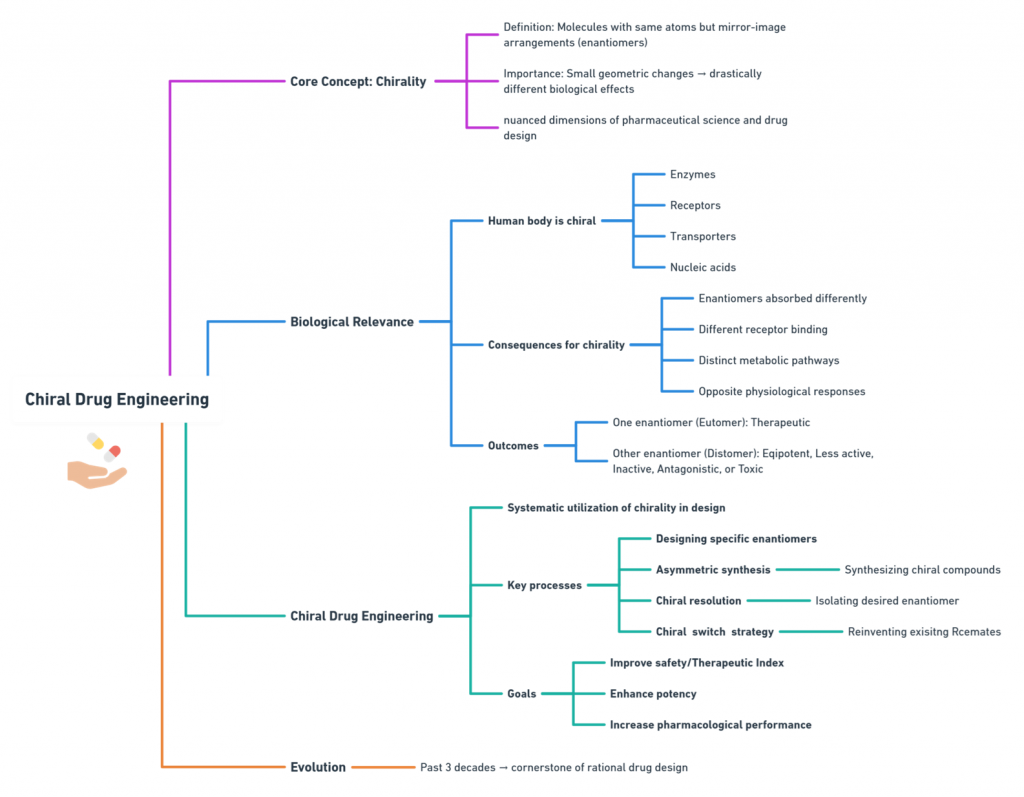
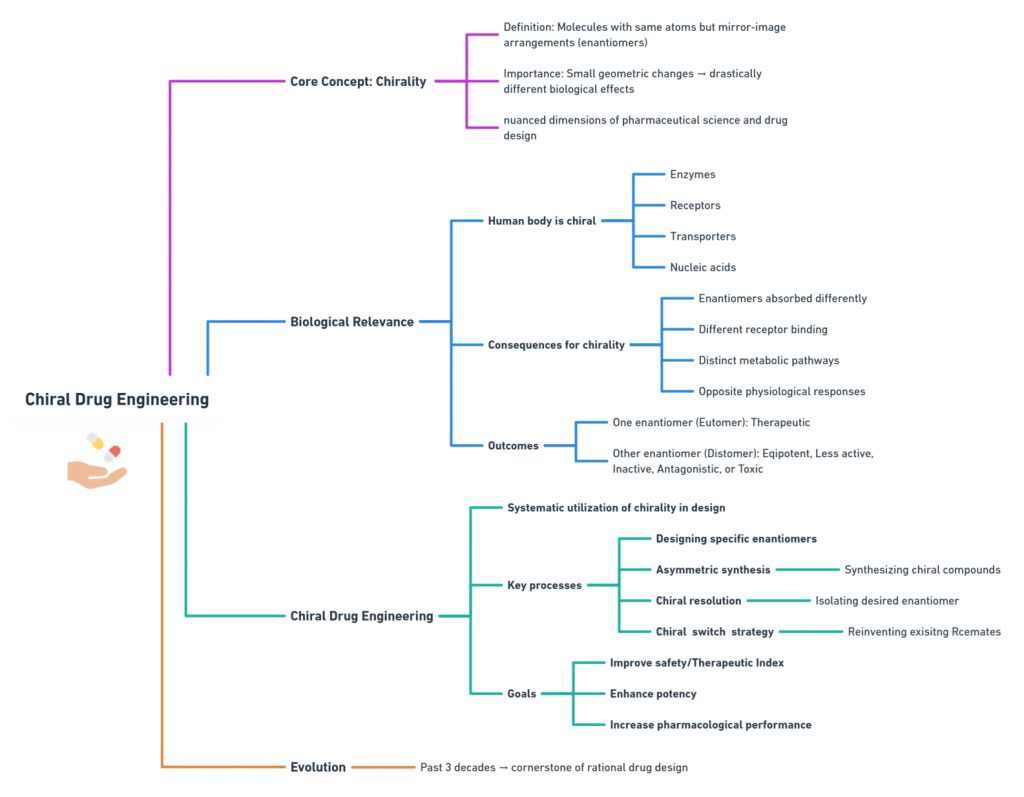
In the world of drug design, a subtle twist in molecular geometry can change everything. Two molecules may share the same atoms and bonding pattern yet behave like entirely different substances simply because they are arranged as non-superimposable mirror images—enantiomers. This property, known as chirality, is one of the most critical yet nuanced dimensions of pharmaceutical science.
The human body—its enzymes, receptors, transporters, and nucleic acids—is inherently chiral. As a result, when a chiral drug enters the body, the left-handed (S- or L-form) and right-handed (R- or D-form) versions may be absorbed differently, bind receptors differently, get metabolized differently, or even trigger opposite physiological responses. One enantiomer may be therapeutic, while the other may be inactive, antagonistic, or in some cases, toxic.
Chiral drug engineering is the systematic approach to harnessing this stereochemical complexity. It involves designing, synthesizing, isolating, and optimizing specific enantiomers—either as single-enantiomer drugs or as chiral switches derived from racemic (50:50) precursors—to improve safety, potency, and performance. Over the past three decades, it has evolved into a cornerstone of rational drug design.
From Racemates to Single Enantiomers: A Paradigm Shift
For much of pharmaceutical history, synthesizing pure enantiomers was difficult, expensive, and technically limiting. As a result, many blockbuster drugs of the 20th century were launched as racemic mixtures—equal parts left- and right-handed molecules. Classic examples include ibuprofen, warfarin, thalidomide, albuterol, and omeprazole.
But with advances in stereoselective chemistry and analytical capabilities, the industry began shifting toward single-enantiomer therapeutics. The motivation was multifold:
- Enhanced safety: Eliminating a harmful or reactive enantiomer.
- Improved efficacy: Delivering only the pharmacologically active molecule.
- Lower dose requirements: Reducing metabolic burden and side effects.
- Patent advantage: Extending product lifecycle via chiral switching.
The 1990s and 2000s marked the era of “chiral switches”—transforming racemic drugs into purified enantiomers. Esomeprazole (from omeprazole), levocetirizine (from cetirizine), escitalopram (from citalopram), and levalbuterol (from albuterol) are textbook examples.
This movement was reinforced by regulatory shifts. The FDA’s 1992 guideline on stereochemical issues recommended enantiomer-specific evaluation, effectively mainstreaming chiral drug engineering.
The Science Behind Enantiomeric Differences in chiral drugs
Understanding enantiomer-specific behavior is central to why chiral drug engineering matters. Suggestion: Open the mind map in a new tab and zoom out to view the complete structure clearly.
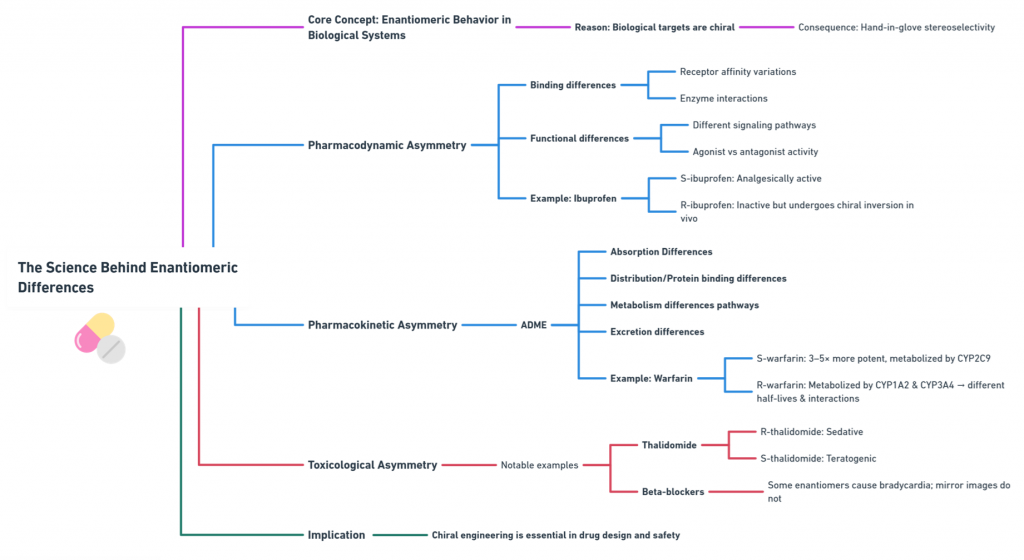
Because biological targets are structurally chiral, giving rise to classic “hand-in-glove” stereoselectivity.
Pharmacodynamic Asymmetry
Enantiomers may:
- bind with different affinities to receptors or enzymes,
- activate different signaling pathways, or
- behave as agonist vs. antagonist pairs.
Example:
S-ibuprofen is the analgesically active form, while R-ibuprofen is largely inactive but undergoes chiral inversion in vivo.
Pharmacokinetic Asymmetry
Enantiomers may differ in:
- absorption rates,
- protein binding estimates,
- metabolism via enantioselective CYP pathways,
- renal or biliary excretion.
Example:
S-warfarin is 3–5 times more potent and metabolized mainly by CYP2C9, while R-warfarin uses CYP1A2 and CYP3A4—leading to dramatically different half-lives and drug interactions.
Toxicological Asymmetry
Some notorious examples highlight this challenge:
- R-thalidomide is sedative; S-thalidomide is teratogenic.
- Certain beta-blocker enantiomers produce bradycardia while their mirror images do not.
These asymmetries justify why chiral engineering is essential, not optional.
Chiral Drug Engineering: The Core Approaches
1. Asymmetric Synthesis: Engineering Chirality at the Molecular Level
Asymmetric synthesis introduces chirality during drug formation rather than separating it afterward. Asymmetric synthesis allows chemists to build molecules in a way that preferentially forms one enantiomer over the other. This field is so impactful that it has been recognized twice by the Nobel Prize in Chemistry—first in 2001 and again in 2021.
The 2001 Nobel Prize in Chemistry was awarded to Ryoji Noyori, William S. Knowles, and K. Barry Sharpless for their pioneering contributions to asymmetric catalysis. Fast-forward twenty years. The 2021 Nobel Prize in Chemistry went to Benjamin List and David W.C. MacMillan for creating asymmetric organocatalysis, a metal-free, environmentally friendly approach to stereoselective synthesis. Together, the 2001 and 2021 Nobel Prizes form the twin pillars of modern chiral drug engineering.
Key Techniques
a. Chiral Catalysts
These catalysts create stereoselectivity without being consumed.
- Chiral metal complexes (e.g., BINAP-Ru complexes in hydrogenations)
- Organocatalysts like proline, cinchona alkaloids, and imidazolidinones
- Biocatalysts (enzymes, engineered variants)
The Noyori asymmetric hydrogenation and Sharpless epoxidation are Nobel-winning breakthroughs that revolutionized the field.
b. Chiral Auxiliaries
Temporary stereochemical guides attached to achiral substrates.
Dissociated after inducing asymmetry.
Example: Evans’ oxazolidinone auxiliaries—widely used for setting stereocenters in β-hydroxy acids and peptides.
c. Stereoselective Photochemical and Electrosynthetic Methods
Emerging fields that use light or electrons to drive enantioselective transformations.
d. Flow-Assisted Asymmetric Synthesis
Continuous flow reactors allow rapid screening, improved heat/mass transfer, and scalable stereoselective processes.
Why it matters
Asymmetric synthesis reduces waste, lowers cost, scalability, avoids inactive/toxic enantiomers, and ensures reproducible stereochemical purity.
Note: For a comprehensive overview of Asymmetric Synthesis, see Chiralpedia’s dedicated blog series <#Asymmetric_Synthesis>.
2. Chiral Resolution: Separating Enantiomers After Formation
When racemates form inevitably or unpredictably, chiral resolution enables isolation of desired enantiomers.
a. Classical Resolution
Treating racemates with chiral acids or bases (e.g., tartaric acid, camphorsulfonic acid) to form diastereomeric salts with different solubilities.
b. Chromatographic Resolution
Using chiral stationary phases (CSPs) in:
- HPLC
- SFC (supercritical fluid chromatography)
- GC (for volatile chiral compounds)
CSPs like amylose, cyclodextrins, or Pirkle phases exploit differential enantiomer–phase interactions.
c. Enzymatic Kinetic Resolution
Enzymes selectively react with one enantiomer.
For example, lipases esterify or hydrolyze a single enantiomer of secondary alcohols.
d. Dynamic Kinetic Resolution (DKR)
Combines racemization of the slow-reacting enantiomer with selective enzymatic conversion—ideally yielding up to 100% theoretical yield.
Note: For expanded discussions on Chiral resolution, explore Chiralpedia’s blog series <#Chiral_Resolution>.
3. Chiral Switch Strategy: Reinventing Existing Racemic Drugs
Chiral switching is both a scientific and commercial strategy.
Why perform a chiral switch?
- Reduce side effects associated with inactive enantiomers
- Improve therapeutic index
- Overcome metabolic variability
- Delay patent expiry (providing market exclusivity)
Successful Case studies
- Esomeprazole (S-omeprazole) from omeprazole
- Escitalopram (S-citalopram) from citalopram
- Levalbuterol (R-albuterol) from albuterol
- Dexlansoprazole, levocetirizine, dexmethylphenidate
Chiral switches often provide clinical advantages significant enough to justify the shift in practice.
Engineering Considerations
- Enantiomer-specific receptor binding
- Differential CYP-mediated metabolism
- Cleaner PK profiles
- Improved safety in special populations
Chiral switches continue to be a fertile ground for innovation where racemic drugs still dominate.
Note: For a detailed treatment of Chiral Switch, visit the Chiralpedia blog series <#Chiral Switch>.
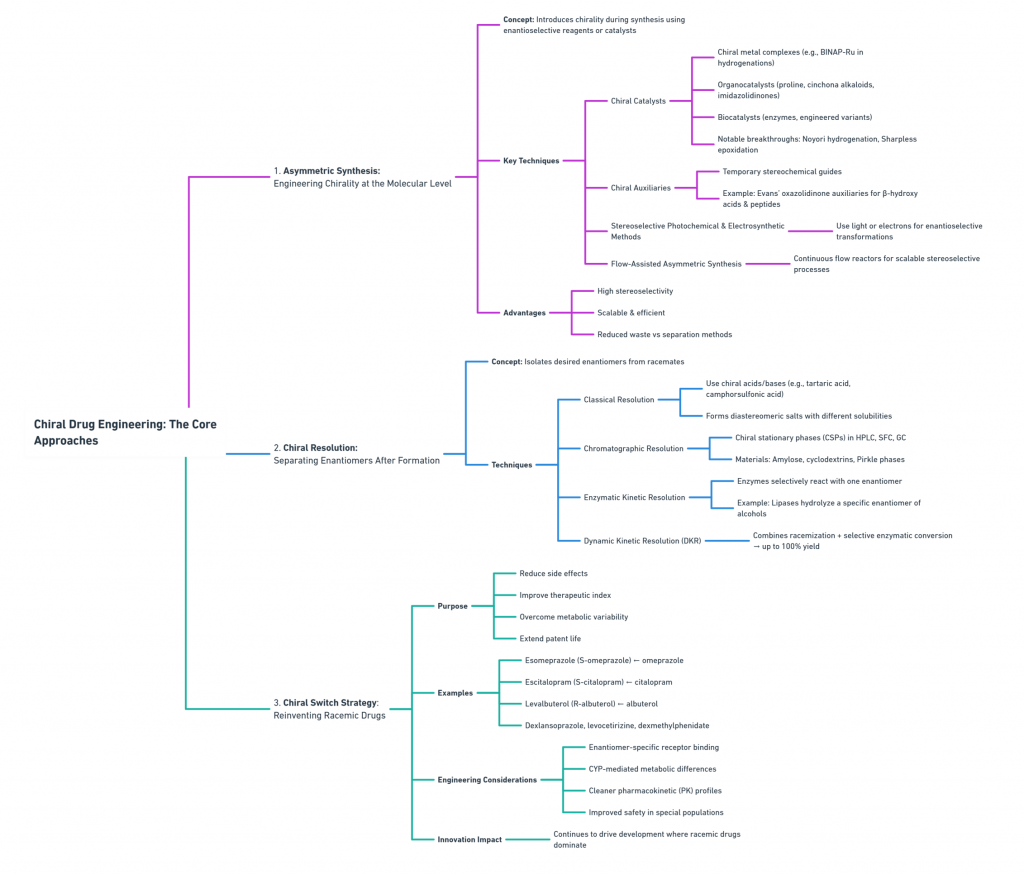
A visual overview of the discussion on Chiral Drug Engineering: The core approaches is presented below as a concept map.
Suggestion: Open the mind map in a new tab and zoom out to view the complete structure clearly.
Engineering Tools and Technologies Driving Modern Chiral Drug Development
1. Computational Chirality Engineering
a. Quantum Chemical Methods
Predict stereochemical outcomes of reactions, transition states, and energetics.
b. AI-Assisted Enantioselective Reaction Prediction
Machine learning models can now suggest optimal catalysts, solvents, and conditions for stereoselective reactions—dramatically reducing experimental load.
c. Molecular Docking and Dynamics
Simulate enantiomer-specific binding to receptors, enabling stereoselective drug design early in the pipeline.
2. Chiroptical and Analytical Technologies
Fast, accurate enantiomeric characterization is the backbone of chiral engineering.
- Chiral HPLC / SFC
- Vibrational Circular Dichroism (VCD)
- Electronic Circular Dichroism (ECD)
- NMR with chiral solvating agents
- Mass spectrometry for chiral metabolites
These tools ensure that every stereocenter is correctly assigned and controlled.
3. Enzyme Engineering and Biocatalysis
Biocatalysis is reshaping stereochemistry.
Genetically engineered enzymes—via directed evolution, CRISPR-based editing, and computational sequence design—achieve remarkable enantioselectivity in:
- hydroxylation
- epoxidation
- reductive amination
- carbon–carbon bond formation
Companies and academic labs now routinely evolve ketoreductases or transaminases specific to a single enantiomer.
4. Flow Chemistry and Process Intensification
Continuous flow platforms enable:
- precise temperature control,
- rapid mixing,
- safer handling of reactive intermediates, and
- scalable stereoselective reactions.
Flow-compatible chiral catalysts and immobilized enzymes further enhance efficiency.
Case Studies: Chirality in Action
1. Esomeprazole
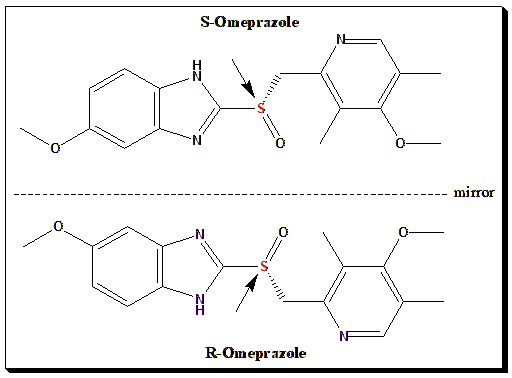
The S-enantiomer of omeprazole provides higher and more consistent bioavailability and stronger acid suppression due to reduced first-pass metabolism.
2. Levofloxacin
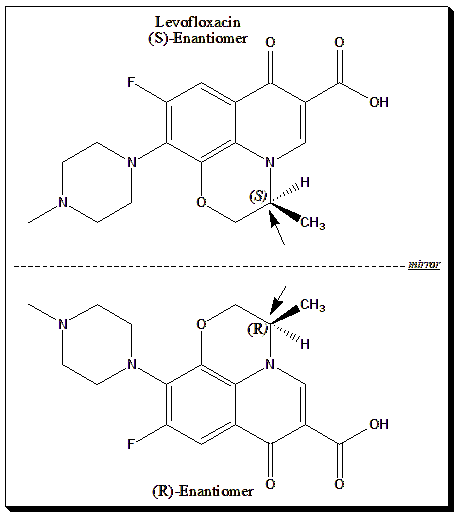
The S-enantiomer of ofloxacin has greater antimicrobial activity and lower toxicity.
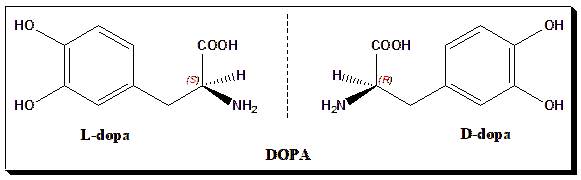
3. L-Dopa
Only the L-enantiomer can cross the blood–brain barrier to treat Parkinson’s disease—one of the earliest triumphs of chirality in medicine.
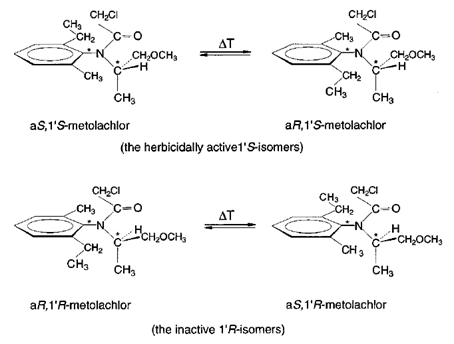
4. (S)-Metolachlor
Outside pharma, this agricultural chiral switch reduced environmental load while improving herbicidal activity. Its chemical structure, containing both central and axial chirality, generates four stereoisomers with markedly different biological properties. S-Metolachlor is not a single pure stereoisomer, but a product enriched in the S configuration at the stereocenter. It contains mostly the two S-configured stereoisomers (S,M) and (S,P).
The Future of Chiral Drug Engineering
The next decade will see rapid growth in:
- AI-driven enantioselective synthesis planning
- Biocatalytic cascades for multi-stereocenter molecules
- Chiral nanomaterials interacting stereoselectively with biological targets
- Stereodivergent catalysts controlling multiple chiral centers simultaneously
- Microbial fermentation for enantiopure API production
The boundary between chemistry, computation, and biology is blurring—and chirality sits at the center of this convergence.
Conclusion
Chiral drug engineering is a prime example of how subtle features of molecular structure can profoundly influence human health. The journey from racemic mixtures to single-enantiomer precision has been shaped by scientific breakthroughs—particularly the Nobel-recognized advances in asymmetric catalysis (2001) and organocatalysis (2021).
By mastering molecular handedness, today’s scientists can design medicines that are more selective, more effective, and safer than ever before. As tools grow smarter and more interdisciplinary, chiral engineering will continue to lead the transformation of modern therapeutics.
References
Nobel Prizes
- The Nobel Prize in Chemistry 2001 – Knowles, Noyori, Sharpless. Chirally Catalyzed Hydrogenation and Oxidation Reactions.
Nobel Foundation. (Press Release, 10 October 2001). - The Nobel Prize in Chemistry 2021 – List, MacMillan. “For the development of asymmetric organocatalysis.”
Nobel Foundation. (Press Release, 6 October 2021).
Asymmetric Catalysis & Organocatalysis
- Noyori, R. (2002). Asymmetric Catalysis: Science and Opportunities. Angewandte Chemie International Edition, 41(12), 2008–2022.
- Knowles, W. S. (2002). Asymmetric Hydrogenations (Nobel Lecture). Angewandte Chemie International Edition, 41(12), 1998–2007.
- Sharpless, K. B. (2002). Searching for New Reactivity (Nobel Lecture). Angewandte Chemie International Edition, 41(12), 2024–2032.
- List, B. (2021). The Development of Asymmetric Organocatalysis (Nobel Lecture). Angewandte Chemie International Edition, 60(36), 18650–18665.
- MacMillan, D. W. C. (2021). The Advent and Impact of Organocatalysis (Nobel Lecture). Angewandte Chemie International Edition, 60(36), 18686–18703.
- https://chiralpedia.com/blog/tag/asymmetric_synthesis/
General Chirality & Single-Enantiomer Drug References
- Nguyen, L. A. (2006). Chiral Drugs: An Overview. International Journal of Biomedical Science, 2(2), 85–100.
- Ariëns, E. J. (1984). Stereochemistry, a Basis for Sophisticated Nonsense in Pharmacokinetics and Clinical Pharmacology.
European Journal of Clinical Pharmacology, 26(6), 663–668. - Agranat, I. D., Caner, H., & Caldwell, J. (2002). Molecular and Clinical Aspects of Chiral Switches: Therapeutic Advantages of Single-Enantiomer Drugs. Nature Reviews Drug Discovery, 1(10), 753–768.
- Calcaterra, A., & D’Acquarica, I. (2018). The Market of Chiral Drugs: Chiral Switches Versus De Novo Enantiopure Compounds.
Journal of Pharmaceutical and Biomedical Analysis, 147, 323–340.
Drug Examples & Stereoselective Pharmacology
- Walle, T. (2000). Stereochemical Differences in the Pharmacokinetics and Pharmacodynamics of Beta-Blockers.
Clinical Pharmacokinetics, 38(2), 79–88. - Andersson, T., et al. (2001). Pharmacokinetics and Pharmacodynamics of Esomeprazole, the S-Isomer of Omeprazole.
Clinical Pharmacokinetics, 40(6), 411–426. - Ward, T. J., & Baker, B. A. (2008). Chiral Separations. Analytical Chemistry, 80(12), 4363–4372.
- Hoffman, R. S. (2001). Warfarin: Pharmacokinetics and Pharmacodynamics of Enantiomers. Journal of Thrombosis and Thrombolysis, 12(1), 7–16.
- Peresypkina, E. V., et al. (2017). Enantioselective Toxicity and Pharmacokinetics of Chiral Drugs. Current Drug Metabolism, 18(7), 608–621.
Biocatalysis & Enzymatic Methods
- Bornscheuer, U. T., et al. (2012). Engineering the Third Wave of Biocatalysis. Nature, 485(7397), 185–194.
- Arnold, F. H. (2019). Innovation by Directed Evolution (Nobel Lecture). Angewandte Chemie International Edition, 58(41), 14420–14426.
Analytical & Chiroptical Methods
- López, C., et al. (2019). Advances in Chiral Chromatography for Pharmaceutical Analysis. TrAC Trends in Analytical Chemistry, 120, 115660.
- Stephens, P. J., Devlin, F. J., & Pan, J.-J. (2008). The Determination of Absolute Configuration Using Vibrational Circular Dichroism (VCD). Chemical Reviews, 108(9), 2854–2871.
Buser, H.-R.; Poiger, T.; Müller, M. D. Enantioselective Determination of Chiral Pesticides in Environmental Matrices Using Enantioselective GC and HPLC. Environmental Science & Technology, 2000, 34 (5), 2690-2696.