“When chemistry tells two stories, the label should tell both”
Drug labels tell us what’s inside — but not always how the molecule twists. And sometimes, that subtle twist changes everything: how a drug acts, how it’s regulated, and even whether it’s safe or addictive.
Chirality — the property of handedness in molecules — has always shaped pharmacology. One mirror image of a molecule (an enantiomer) can save lives, while its twin may do little or even harm. Yet, despite decades of stereochemical awareness, most drug labels still fail to tell the full chiral story. This disconnect between molecular science and clinical communication is what we call the enantiomer gap.
And behind that enantiomer gap lies a deeper regulatory blind spot — where the science of stereochemistry advances far faster than its reflection in labelling, approval, and clinical communication. Regulations often treat racemates as single entities, even when the underlying enantiomers behave as distinct drugs in terms of efficacy, metabolism, and toxicity. As a result, what begins as a scientific nuance can turn into a communication gap — one that affects how prescribers, regulators, and patients understand the very drugs they rely on.
Case Study 1: When racemates hide in plain sight
Take Propranolol, one of the most widely used β-blockers in medicine. The prescribing information simply lists “propranolol hydrochloride,” with no mention of chirality. To most clinicians, that seems sufficient — after all, the dose works.
But behind that label lies a fascinating asymmetry.
Propranolol exists as two mirror images: (S)-propranolol, which provides almost all the β-adrenergic blockade, and (R)-propranolol, which contributes little to the therapeutic effect. Research has shown that the metabolism and pharmacodynamics of these enantiomers differ significantly — e.g., the S(-) form is more active and the R(+) form is cleared differently.
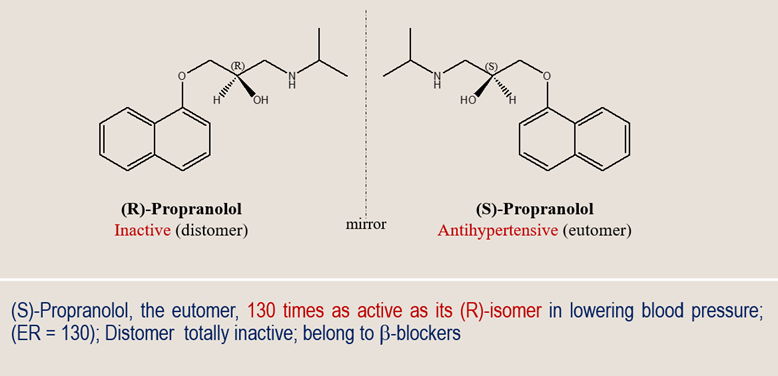
The marketed drug is a racemic mixture, containing equal parts of both. The dosing was empirically optimized for this racemate, so the label functions clinically. Yet, the enantiospecific information — the deeper story of which “hand” actually works — remains invisible to most readers of the label.
This is not an isolated case. Dozens of racemic drugs — from ibuprofen to verapamil — have active and inactive (or differently active) enantiomers. Some of them have later been “chiral-switched” (reformulated as single-enantiomer drugs) but many remain racemates on the market.
Case Study 2: When regulators recognize the twist
Sometimes, regulators and clinicians do take note — especially when safety is on the line.
Consider Methadone, long used in pain management and opioid-dependence therapy. It, too, is chiral, consisting of (R)-methadone and (S)-methadone.
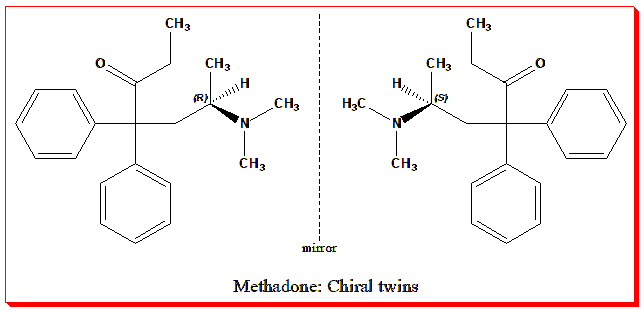
- (R)-methadone provides the desired opioid analgesic and anti-addictive effects (via the μ-opioid receptor).
- (S)-methadone, on the other hand, does not significantly contribute to analgesia but can prolong the QT interval (a measure of cardiac repolarization) and thus increase arrhythmia risk.
Unlike propranolol, methadone’s stereochemistry has entered regulatory awareness. The FDA’s 1992 (U.S. Food and Drug Administration) guidance on stereoisomeric drugs emphasized that “when stereoisomers are biologically distinguishable, they might seem to be different drugs” and thus require appropriate characterization in development. Many guidelines now explicitly note that R-methadone is the active isomer and some jurisdictions allow or recommend compounding R-methadone-only formulations to reduce cardiac risk.
This recognition represents an important step toward enantiospecific pharmacovigilance — where drug safety and efficacy are evaluated not just for a chemical name, but for the molecular “hand” that truly matters.
Case Study 3: When mirror molecules live double lives
If propranolol hides chirality and methadone acknowledges it, the methorphan enantiomers take the story one step further: they embody chirality’s power to divide entire regulatory worlds.
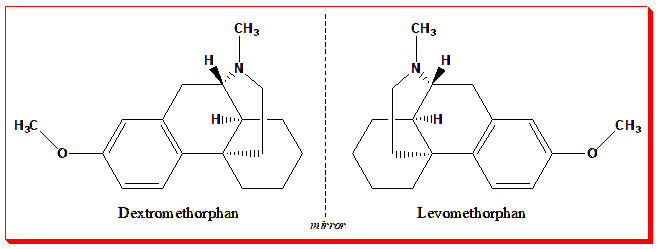
The methorphan skeleton gives rise to two enantiomers with identical formulas but opposite configurations:
- Dextromethorphan (d-isomer) is sold over-the-counter as a cough suppressant and mild NMDA receptor antagonist.
- Levomethorphan (l-isomer) is a Schedule II controlled opioid analgesic, potent, addictive, and tightly regulated.
Same molecular skeleton, opposite spatial arrangement — yet their labels, scheduling, and control differ entirely. Chirality here defines not just pharmacology but legal identity.
It’s difficult to find a clearer demonstration that chirality determines not just how a molecule interacts with biology, but how it interacts with the law and the public health system.
The “enantiomer gap” — and why it matters
Across these examples — propranolol, methadone, and the methorphan pair — a consistent theme emerges: chirality is everywhere, but chiral awareness is uneven.
- In some cases, chirality is ignored, hidden within a racemic name.
- In others, it is acknowledged, shaping clinical safety and dosing.
- And sometimes, it defines entirely separate drugs and legal frameworks.
This unevenness forms the enantiomer gap — a space where scientific precision outpaces regulatory and educational communication. When drug labels omit stereochemical details, they inadvertently teach a simplified, sometimes misleading, view of drug action.
Pharmacology students may learn about receptor binding and ADME (absorption-distribution-metabolism-excretion) profiles without realizing that the molecule’s handedness is often the silent determinant of all those properties. Clinicians, too, may assume that a drug name reflects a single active entity, when in fact it may represent two competing or complementary chiral species.
The FDA’s 1992 “Development of New Stereoisomeric Drugs” guidance formalized this issue, emphasizing that regulatory submissions must identify stereochemical composition, assess enantiomer-specific pharmacologic/ toxicologic properties, and justify whether racemate or single enantiomer is appropriate.
Why enantiospecific labelling matters
Why does this matter in practice?
Because chirality can determine not just how a drug works, but whether it’s safe.
An inactive or weak enantiomer may dilute efficacy — requiring higher doses or increased exposure. A toxic one can generate side effects or even trigger withdrawals, as seen historically with chiral disasters (e.g., the thalidomide tragedy). Understanding which enantiomer is responsible for which effect helps refine therapeutic windows and anticipate adverse risks.
For pharmacologists and medicinal chemists, this is obvious. But for clinicians and patients, labelling is the interface — the point where chemistry meets care. If the label doesn’t reflect the chiral reality, critical context can be lost.
Enantiospecific labelling can also inform pharmacovigilance. When adverse events occur, knowing whether a racemate or enantiopure formulation was used can help pinpoint causes. The same logic applies to bioequivalence testing, where generic formulations of chiral drugs must demonstrate stereochemical consistency with the reference product.
Toward enantiospecific transparency
How can we move closer to that goal?
A few key steps could make a significant difference:
- Label clarity: Include the chiral form — e.g., “(S)-propranolol hydrochloride” or “R-methadone hydrochloride” — wherever it is clinically relevant.
- Pharmacological annotation: In product monographs/summaries of product characteristics (SPCs), explicitly mention which enantiomer contributes to therapeutic action and which may contribute to toxicity.
- Educational integration: Pharmacy curricula, medicinal chemistry courses and clinical teaching should emphasise enantiospecific pharmacology early, using case studies like those above to connect chemistry and clinical relevance.
- Post-market monitoring: Pharmacovigilance systems could track adverse effects separately for racemic and single-enantiomer formulations, guiding safer prescribing decisions.
By fostering such transparency, we not only improve patient safety but also align drug communication with molecular reality.
Why Chiralpedia cares
At Chiralpedia, our mission is to make chirality visible — in classrooms, in research, and in the real world of medicines. The enantiomer gap is not simply a technical issue; it reflects a broader need for chemical literacy in healthcare. When a label misses the twist, understanding stops at the surface. When a label names the twist, chemistry and medicine reconnect.
Chirality is not an esoteric detail — it is the grammar of molecular behavior. Recognizing it in drug labelling is one way to make that grammar readable to everyone who prescribes, dispenses, or studies medicines.
So the next time you see a familiar name on a label — propranolol, methadone, dextromethorphan — take a closer look. Behind that name may lie a twist that changes everything.
Further Reading
Food and Drug Administration (FDA). Development of New Stereoisomeric Drugs (FDA Policy Statement). 1992, Fed Reg 57(222): 53640-53645.
Mehvar R, Brocks DR. Stereospecific pharmacokinetics and pharmacodynamics of beta-adrenergic blockers in humans. J Pharm Pharm Sci. 2001 May-Aug;4(2):185-200.
Coelho MM, Fernandes C, Remião F, Tiritan ME. Enantioselectivity in Drug Pharmacokinetics and Toxicity: Pharmacological Relevance and Analytical Methods. Molecules. 2021 May 23;26(11):3113. doi: 10.3390/molecules26113113.
Taha Ahmad, Monica A. Valentovic, Gary O. Rankin, Effects of cytochrome P450 single nucleotide polymorphisms on methadone metabolism and pharmacodynamics. Biochemical Pharmacology, 153, 2018, 196-204.
Roger A, Sheldon, Chirotechnology: industrial synthesis of optically active compounds, Marcel Dekker,1993.
Chiral drugs. Wikipedia, Wikipedia Foundation, 09/10/2022. https://en.wikipedia.org/wiki/Chiral_drugs and references therein
Ariëns, Everardus J. (1986). “Stereochemistry: A source of problems in medicinal chemistry”. Medicinal Research Reviews. 6 (4): 451–466. doi:10.1002/med.2610060404
Ariëns, E. J. (1984). “Stereochemistry, a basis for sophisticated nonsense in pharmacokinetics and clinical pharmacology”. European Journal of Clinical Pharmacology. 26 (6): 663–668. doi:10.1007/BF00541922