“Decoding the rules: how chemists name and navigate molecular twists.”
Introduction
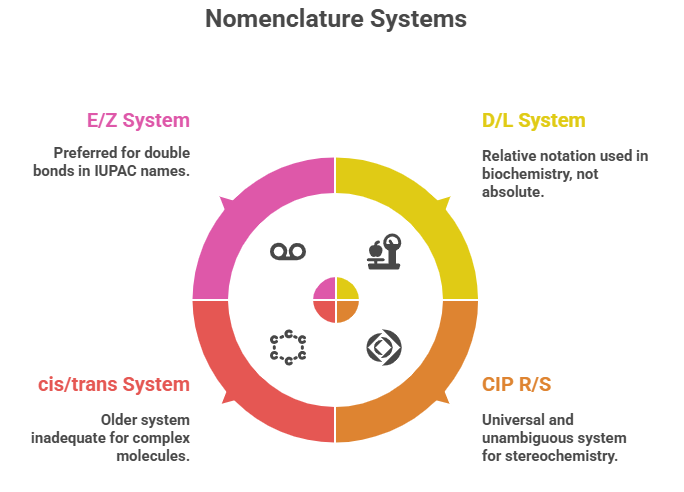
Correctly describing the stereochemistry of a molecule is as important as understanding it. In this part, we focus on the Cahn–Ingold–Prelog (CIP) system, which provides the rules for unambiguous assignment of absolute configuration at stereocenters (R/S) and for double bond geometry (E/Z) system. We will outline the CIP priority rules step-by-step and demonstrate how to apply them to pharmaceutical molecules. Additionally, we will discuss older nomenclature systems (like D/L and cis/trans) in passing and why CIP is the preferred standard. By using examples from drug molecules, we’ll see how stereochemical nomenclature is used in practice (e.g., distinguishing (R)- from (S)-thalidomide, or E- from Z-tamoxifen). A visual flowchart for R/S assignment will be suggested as a learning tool to accompany the textual rules.
CIP Priority Rules – Assigning R and S
The CIP system, devised by chemists R. S. Cahn, C. K. Ingold, and V. Prelog in the mid-20th century, is endorsed by IUPAC for specifying absolute configurations of chirality centers. Each stereocenter is assigned either R (rectus, Latin for right) or S (sinister, Latin for left) according to the following sequence of rules:
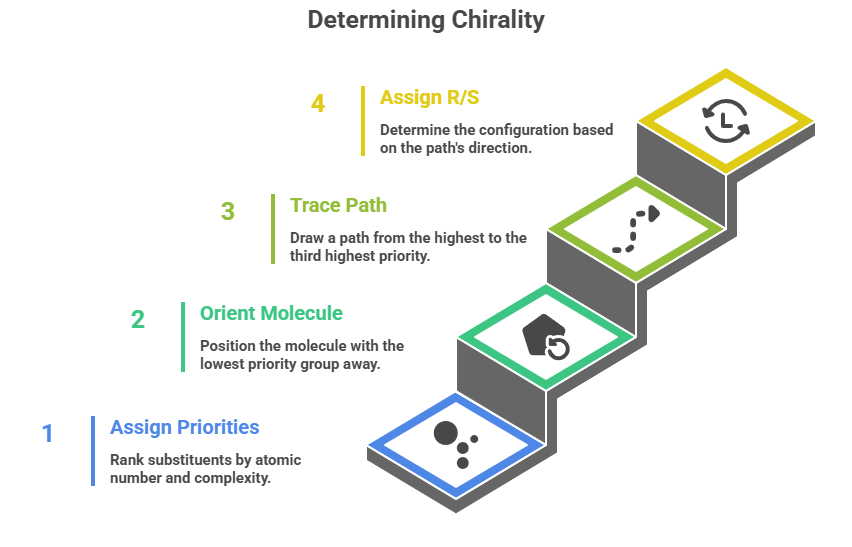
- Assign Priority to Substituents: Look at the four atoms directly bonded to the chiral center. Rank them by atomic number: the higher the atomic number, the higher the priority. For example, consider a carbon with substituents H, OCH3, NH2, and CH3: Oxygen (atomic #8) takes highest priority, then nitrogen (#7), then carbon (#6, the methyl), and hydrogen (#1) is lowest. If two substituents begin with the same atom type, move outward along the chain to find the first point of difference (the first place where the groups differ in atomic number). For instance, between an ethyl (–CH2CH3) and a methyl (–CH3) substituent: the first atom in each is carbon (tie), then compare the next set of attached atoms – ethyl has another carbon attached (next to the first carbon), whereas methyl has only hydrogens. Carbon outranks hydrogen, so ethyl wins over methyl. Double or triple bonds are treated as if the atom is bonded to multiple phantom atoms: e.g., C=O is considered as if carbon is attached to two oxygen atoms for priority purposes. Isotopes: the heavier isotope gets higher priority (e.g., T (tritium) > D (deuterium) > H).
- Orient the Molecule: Once priorities 1 (highest) through 4 (lowest) are assigned, mentally orient the molecule (or physically with a model) so that the lowest priority group (4) is pointing away from you (i.e., its bond to the chiral center is directed into the page). In wedge-dash terms, that usually means the lowest priority should be on a dashed bond if possible. If the lowest priority substituent is not conveniently placed away, one trick is to make a mental (or drawn) swap or rotate the molecule accordingly – but be cautious: swapping two groups on a stereocenter inverts its configuration, so if you do an odd number of swaps to get the low priority in back, you must invert your result at the end. A reliable method is to use a molecular model or draw a Fischer projection (with lowest priority on a vertical line, which points away in Fischer convention.
- Trace a Path from 1→2→3: With the lowest priority behind, draw or imagine an arrow that goes from priority 1 substituent to priority 2 to priority 3 in order. Do this in the plane of the page (the chiral center is the vertex where substituents attach).
- Assign R or S: If the path 1–2–3 is clockwise, the center is R (think “Right-turn = Rectus”). If the path is counter-clockwise, the center is S. An easy mnemonic: imagine a clock dial facing you; if you go from 12→3→6, that’s clockwise (R). Or use the latin: rectus means right, which on a compass is East, and East on most maps is to the right – a stretch, but it helps some students.
Note: For a detailed discussion checkout following blog @<https://chiralpedia.com/blog/naming-enantiomers-the-r-s-system/>.
Case Study 1: Thalidomide
For example, let’s apply this to a simple pharmaceutical molecule: thalidomide. Thalidomide has one chiral center (at the glutaramide ring carbon). Assigning CIP priorities: substituents are (1) the phthalimido group (which begins with a Nitrogen attached to two carbonyls – treat as N attached to two “O” phantom atoms, giving that carbon a high priority), (2) the amide carbonyl group (C attached to O, etc.), (3) the adjacent CH2 group, and (4) hydrogen. Without going into exhaustive detail, if we do the exercise, one can determine one enantiomer is (R)-thalidomide and the other (S)-thalidomide.
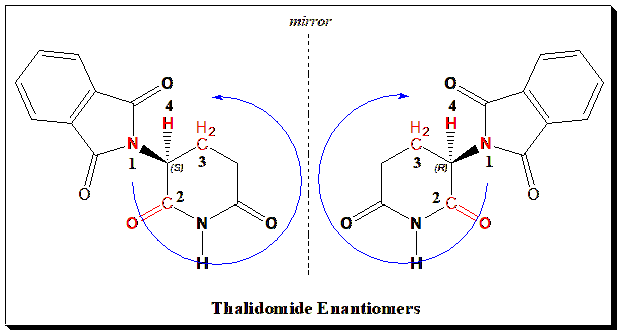
A critical point: R and S are labels for absolute configuration. They do not tell us which enantiomer rotates light which way – that must be determined experimentally or by correlation with known standards. For instance, (R)-thalidomide is often cited as the teratogenic one and (S)-thalidomide as the sedative, but in reality thalidomide racemizes in vivo (see Part 6).
Case Study 2: Ibuprofen
As another example, the anti-inflammatory drug ibuprofen is marketed as a racemate, but the active eutomer is S-(+)-ibuprofen. Here S- is the CIP absolute configuration and “+” indicates the direction of optical rotation of that enantiomer in solution. The other enantiomer is R-(–)-ibuprofen, which is weaker as an analgesic but partly converted to S in the body. This convention of prefixing a molecule name with (R)- or (S)- (and if relevant, (+) or (–)) is ubiquitous in medicinal chemistry literature to precisely specify which stereoisomer is being discussed.
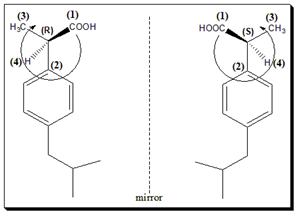
Priority assigned for substituents as per CIP convention
E/Z for Double Bonds
In addition to chiral centers, stereochemistry can arise from double bonds (geometric isomerism). The CIP system extends to assign E (entgegen, German “opposite”) or Z (zusammen, “together”) for C=C bonds, replacing the older “cis/trans” notation when there are more than two different substituents involved. Here’s how to do it:
- For a given double bond, consider each double-bonded carbon separately. Apply CIP priority rules to the two substituents on that carbon. Do the same for the other carbon. Now you’ve identified a “higher priority” and “lower priority” substituent on each end of the C=C.
- If the two higher priority substituents are on the same side of the double bond (i.e., both “up” or both “down” in a planar depiction), the double bond configuration is Z (“zusammen”). If they are on opposite sides (one “up” and one “down”), it’s E (“entgegen”).
Read more @ the following blog article <https://chiralpedia.com/blog/cis-trans-and-e-z-notation-choose-your-side/>
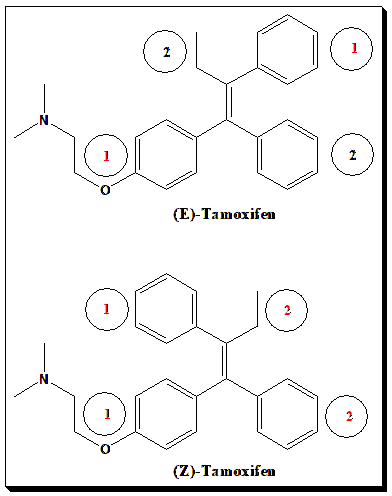
Case Study: Tamoxifen
The drug tamoxifen, a selective estrogen receptor modulator. Tamoxifen has a C=C in its structure (in the triphenylethylene core). If we assign priorities on each end (the substituted phenyl vs. H on one end, and two different substituted aryl groups on the other), one finds that tamoxifen’s active form is the E isomer (often loosely called trans-tamoxifen). The Z isomer (cis) is inactive as an ER modulator. In simpler cases where each carbon has one H, cis/trans and E/Z coincide (cis = Z, trans = E). But for clarity, especially in polysubstituted alkenes, E/Z is used.
Case Study: Retinoic acid
Another pharmaceutical example: retinoic acid exists as all-trans (tretinoin) and 13-cis (isotretinoin) forms. Here, because there are multiple double bonds, a full E/Z designation can be given to each, but they are commonly known by cis/trans in reference to the terminal double bond. Tretinoin (all-E-retinoic acid) and isotretinoin (13-cis-retinoic acid) have different therapeutic uses (tretinoin for leukemia and dermatology; isotretinoin for acne) and different safety profiles, illustrating the importance of double bond stereochemistry.
Cis-trans Isomerism due to Double bonded carbon-heteroatom systems
For a discussion on Cis-trans isomerism due to double bonded carbon–heteroatom systems (oximes and azo compounds) refer to our earlier blog on “Cis-trans and E-Z notation: choose your side” @ <https://chiralpedia.com/blog/cis-trans-and-e-z-notation-choose-your-side/>.
Example – Nomenclature in Drug Names
If you read the full chemical name of a drug in a scientific insert or regulation, it often encodes stereochemistry. For instance, the anticoagulant clopidogrel is marketed as the single stereoisomer (the SR diastereomer, with absolute configuration (S) at one center and (R) at another – sometimes denoted as (–)-clopidogrel in older literature). Its IUPAC name includes both stereo-descriptors. As another example, esomeprazole – as mentioned earlier – is the S-enantiomer of omeprazole. In publications you might see it named “(S)-omeprazole”. The FDA label even refers to esomeprazole magnesium trihydrate as “the S-isomer of omeprazole” (hence the brand name Nexium’s implication as the next-generation single-isomer). The ability to decode R/S and E/Z in drug names allows a chemist or pharmacist to understand which stereochemical form is present. Regulatory authorities often require that the absolute configuration be determined and indicated for chiral drugs – for example, the FDA’s 1992 policy states absolute stereochemistry should be established early in development.
Fischer vs. CIP (D/L system)
Before CIP, sugars and amino acids were assigned D or L configurations relative to a reference (glyceraldehyde). This D/L system is still used in biochemistry (e.g., L-amino acids, D-glucose) but it is a relative notation, not an absolute one except by historical convention. For instance, natural L-amino acids are usually (S) by CIP rules (with the notable exception of cysteine which is (R) due to sulfur’s priority). The D/L system does not generalize well beyond certain families of compounds and is not used for most pharmaceuticals (except those directly derived from sugars or amino acids where tradition persists). CIP R/S is universal and unambiguous. Similarly, the older cis/trans for rings or alkenes becomes inadequate when more substituents are involved or in complex rings – hence E/Z is preferred for double bonds in IUPAC names. In rings, terms like cis/trans are still used (e.g., cis-atracurium), but often one can specify configurations at each stereocenter for clarity.
Summary (Part 3)
– The CIP system provides a rigorous method to assign absolute configuration (R or S) to chiral centers and geometric configuration (E or Z) to double bonds.
– Priority rules: Based on atomic number (and connectivity for ties); these rules are applied to determine ordering of substituents.
– Assigning R/S: After ranking, orient the molecule with the lowest priority substituent away and see if the 1-2-3 sequence goes clockwise (R) or counter-clockwise (S). This gives the absolute stereochemistry of that center.
– Assigning E/Z: Determine high-priority substituent on each double bond carbon, then check positions – same side = Z, opposite sides = E. Useful for specifying alkene isomers unambiguously, especially when cis/trans nomenclature fails.
– Pharmaceutical examples illustrate use of this nomenclature: (S)-omeprazole vs racemic omeprazole, (R,R)-formoterol vs (S,S)-formoterol (enantiomeric pairs of a bronchodilator, where only one is marketed), etc.
– Why it matters: Precise stereochemical nomenclature avoids ambiguity – critical in drug patents, regulatory filings, and medicinal chemistry publications. It tells chemists exactly which stereoisomer is present or being made, which is crucial given that different stereoisomers can have very different properties. Regulatory agencies expect that sponsors characterize the stereochemistry of chiral drugs and use the proper nomenclature in documentation.
– Older nomenclatures: D/L and cis/trans are either limited or context-dependent and have largely been superseded in formal use by CIP descriptors for clarity, though you will still encounter them (especially D/L in biochemistry).
Suggested Reading
IUPAC Rules (1976, 1982, etc.) for Stereochemical Nomenclature – the foundational papers by Cahn, Ingold, Prelog (e.g., Angew. Chem. Int. Ed., 1966, 5, 385) detailing the CIP system. (Classic primary literature on CIP rules.)
IUPAC Blue Book (Nomenclature of Organic Chemistry), latest edition – section on stereo-descriptors (R/S and E/Z). (The official nomenclature manual with examples.)
“Sequence Rules for Specifying Configuration (R/S)” – Chemistry LibreTexts section. (A tutorial-like explanation with interactive examples.) – https://chem.libretexts.org/Courses/Purdue/Chem_26505%3A_Organic_Chemistry_I_(Lipton)/Chapter_3._Stereochemistry/3.6_Cahn-Ingold_Prelog_Rules#:~:text=If%20there%20are%20two%20substituents,chain%20has%20the%20higher%20priority
FDA Guidance 1992 – Appendix or relevant part where they emphasize knowing absolute stereochemistry[46]. (Regulatory angle on importance of correct stereochemical assignment.)
Hinchliffe, A. (2018). Chemical Nomenclature (Chapman & Hall): Chapter on stereochemical nomenclature. (A concise guide with many examples, covering CIP, and also discussing pitfalls and special cases like atropisomers, etc.)
https://en.wikipedia.org/wiki/Chiral_drugs