“Approval is not the end of a molecule’s journey – the mirror must be watched, studied, and questioned throughout its life.”
Introduction
The development and approval of chiral drugs, especially single-enantiomer entities, marked a new chapter in pharmaceutical science. However, approval is only the beginning. Ensuring that a chiral drug continues to behave safely and predictably in the real world demands vigilant post-marketing surveillance, adaptive regulatory frameworks, and constant scientific reevaluation.
In this final episode, we explore the lifecycle management of chiral drugs post-approval:
- How do pharmacovigilance systems monitor chiral-specific safety issues?
- What regulatory mechanisms exist to detect late-emerging risks?
- How might future chiral drug development evolve toward better transparency, efficiency, and personalization?
The mirror image metaphor reminds us: molecules that once seemed well understood can reveal hidden faces over time.
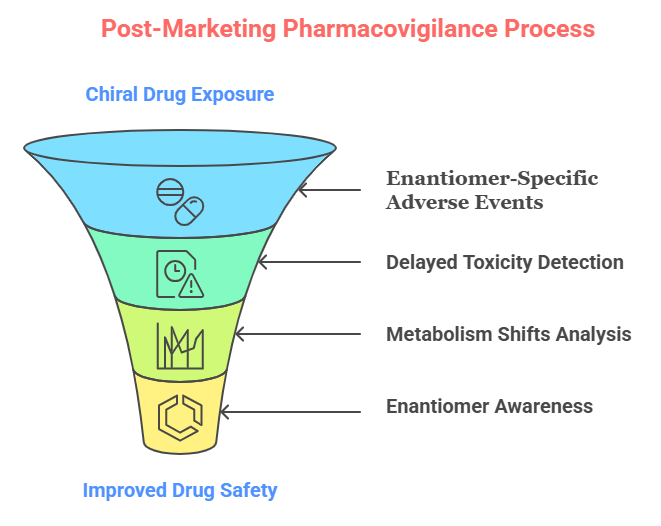
Post-Marketing Pharmacovigilance for Chiral Drugs
Pharmacovigilance refers to the science and activities relating to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problems.
For chiral drugs, specific considerations include:
1. Enantiomer-Specific Adverse Events
While both enantiomers are usually studied during development, rare or subtle enantiomer-specific adverse events may not emerge until large-scale post-marketing exposure.
Examples:
- If the distomer is minimally active but bioaccumulates over time, delayed toxicity could occur.
- If stereoselective metabolism shifts in certain populations (e.g., genetic polymorphisms), one enantiomer could unexpectedly dominate, altering therapeutic outcomes.
Thus, pharmacovigilance must remain enantiomer-aware (EMA, 2005).
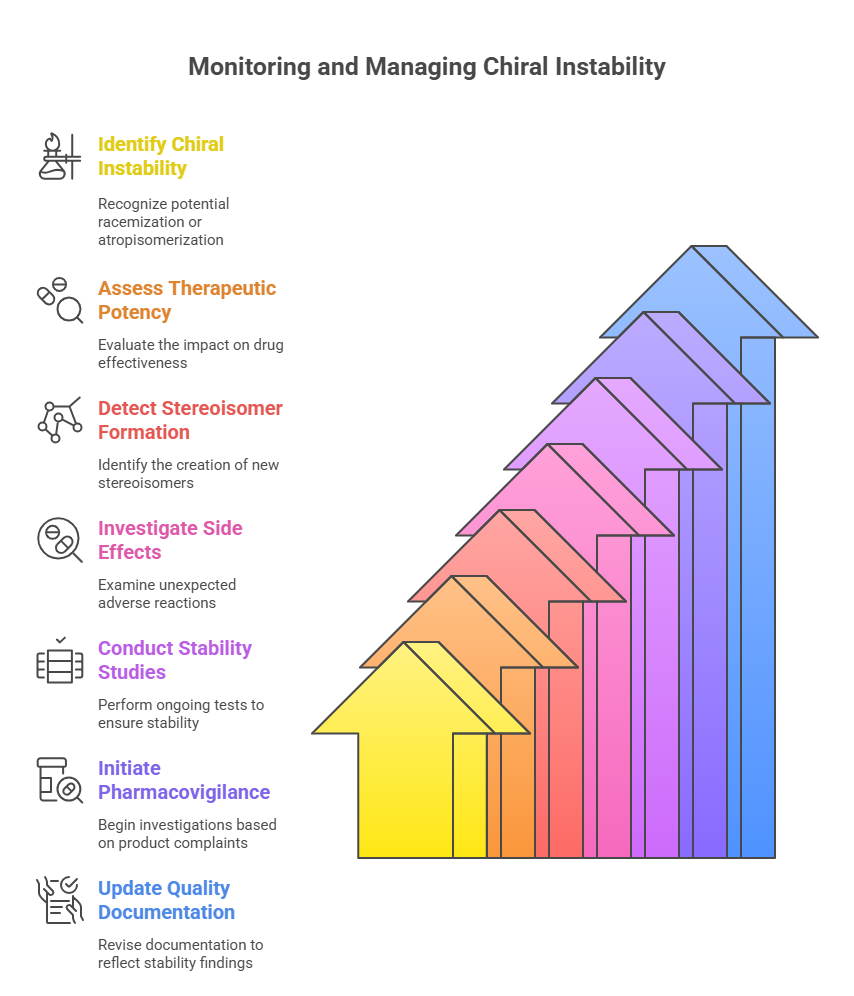
2. Monitoring for Chiral Instability
Dynamic racemization or atropisomerization may lead to:
- Loss of therapeutic potency.
- Formation of untested stereoisomers.
- Unexpected side effects.
Stability-indicating assays must be incorporated into:
- Ongoing stability studies.
- Pharmacovigilance investigations when product complaints arise.
Regulators expect manufacturers to update quality documentation if significant instability is detected post-approval (ICH, 2003).
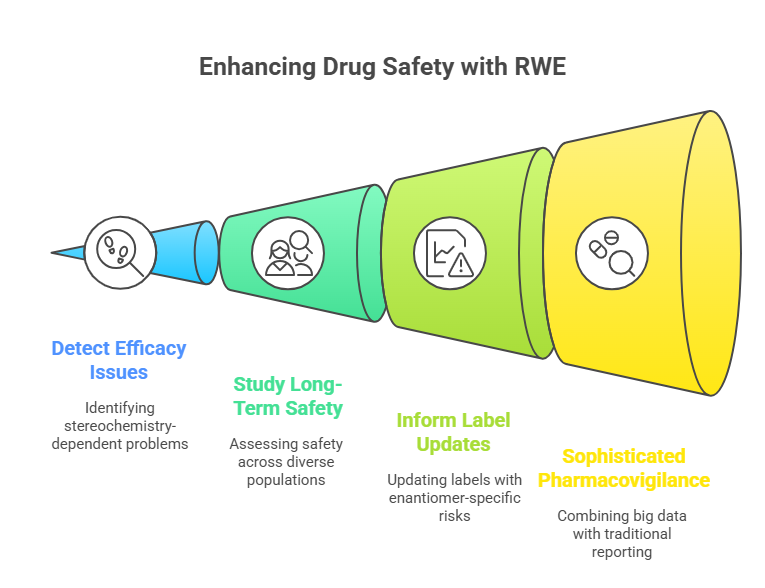
3. Real-World Evidence (RWE) and Chiral Drugs
RWE -data collected from routine clinical practice -offers powerful tools for:
- Detecting stereochemistry-dependent efficacy issues.
- Studying long-term safety across diverse populations.
- Informing potential label updates regarding enantiomer-specific risks.
As databases grow, enantiomer-specific pharmacovigilance will become more sophisticated, combining big data analytics with traditional post-marketing reporting (FDA, 2018).
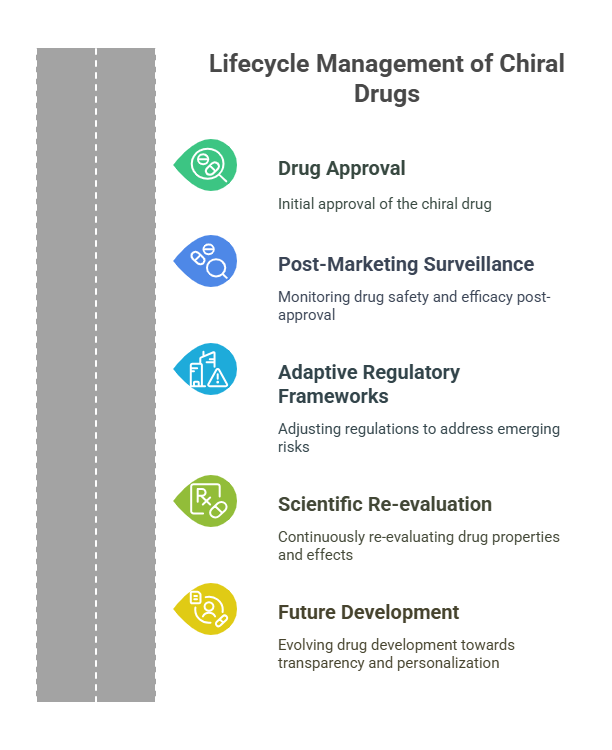
Lifecycle Management of Chiral Drugs
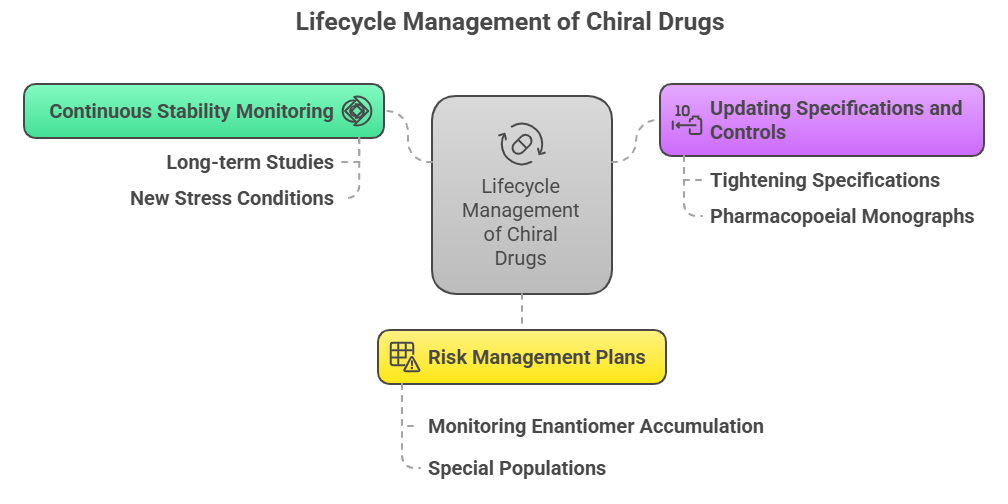
Beyond initial approval, maintaining the safety and efficacy of chiral drugs requires proactive lifecycle strategies:
1. Continuous Stability Monitoring
- Long-term real-time stability studies must explicitly assess chiral integrity.
- New stress conditions (e.g., new storage temperatures) may reveal hidden vulnerabilities.
2. Updating Specifications and Controls
- If enantiomeric ratios shift over time, release specifications may need tightening.
- Pharmacopoeial monographs for chiral drugs often evolve to incorporate improved methods for enantiomeric purity testing.
3. Risk Management Plans (RMPs) for Chiral-Specific Risks
- Modern approvals often include RMPs focused on chiral aspects:
- Monitoring for signs of unexpected enantiomer accumulation.
- Special populations (e.g., liver disease patients with impaired stereoselective metabolism).
These RMPs align with ICH E2E guidance on pharmacovigilance planning (ICH, 2005).
Learning from the Past: Case Studies in Chiral Pharmacovigilance
Thalidomide: A Perpetual Warning
The tragic lessons of thalidomide remind us that even subtle stereochemical changes -here, in vivo racemization -can have catastrophic consequences if ignored post-approval (McBride, 1961).
Esomeprazole: Successful Lifecycle Stewardship
Post-approval monitoring confirmed the stability and consistent performance of esomeprazole across global markets, reinforcing its therapeutic positioning (Andersson, 2001).
The Future: Innovations in Chiral Pharmacovigilance
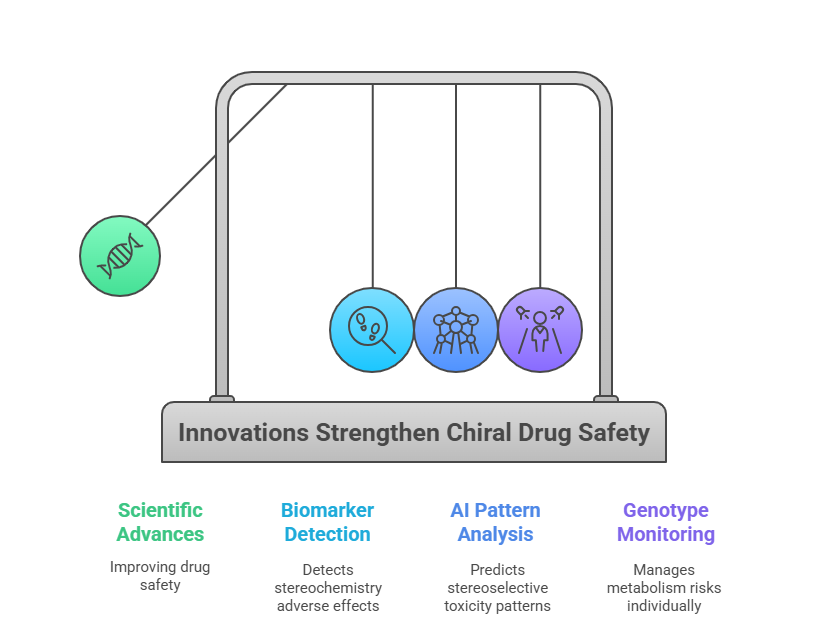
Several scientific advances promise to strengthen the post-marketing safety of chiral drugs:
1. Chiral-Specific Biomarkers
- Identifying biomarkers sensitive to enantiomeric imbalance could allow early detection of stereochemistry-dependent adverse effects.
2. AI and Machine Learning
- Predictive models could analyze post-marketing data for patterns suggesting stereoselective toxicity or loss of efficacy.
- AI could assist in automating detection of chiral-specific safety signals hidden in large datasets (Rajkomar et al., 2019).
3. Personalized Pharmacovigilance
- Genotype-based pharmacovigilance could anticipate and manage stereoselective metabolism risks in individuals (Zhou, 2011).
Toward a Future of Ethical, Transparent, and Patient-Centric Chirality
Chiral drug development and regulation have made extraordinary progress. Yet, continued vigilance is essential:
- Transparency about enantiomeric properties must persist across the drug lifecycle.
- Ethical stewardship of chiral innovations must prioritize patient welfare over commercial imperatives.
- Scientific curiosity must continue to challenge assumptions and investigate the hidden dimensions of molecular asymmetry.
Ultimately, the regulatory odyssey of chiral drugs reflects broader truths about medicine:
What seems simple often harbors unseen complexities.
What we control today must be watched tomorrow.
Conclusion
The story of chiral drugs -from regulatory neglect to sophisticated vigilance -is a triumph of scientific learning, ethical introspection, and global collaboration. As chemistry, biology, and data science converge, the future promises even more precise, safer, and patient-centered applications of chirality. But no matter how advanced our technologies or comprehensive our regulations become, the mirror image principle remains: The true nature of things often reveals itself only when viewed from all sides -patiently, humbly, and continuously.
📖 Series Summary and Final Reflection
Over these ten episodes, we have traced the extraordinary evolution of chiral drug regulation:
- From the ignorance of stereochemistry before the 1990s.
- Through the transformative regulatory shifts of the 1990s.
- Across global agency differences, technological revolutions, scientific debates, and ethical-economic dilemmas.
- Into the emerging challenges of complex chirality and the unending need for vigilance.
The regulatory odyssey of chiral drugs mirrors our own: a journey from simplicity to complexity, from confidence to humility, from isolated insights to holistic understanding. The story -like the molecules themselves -continues.
References
Andersson, T. (2001). Pharmacokinetics, metabolism and interactions of acid pump inhibitors: Focus on omeprazole, lansoprazole, pantoprazole, and rabeprazole. Clinical Pharmacokinetics, 40(7), 523–538.
European Medicines Agency (EMA). (2005). Guideline on Risk Management Systems for Medicinal Products for Human Use (EMEA/CHMP/96268/2005).
Food and Drug Administration (FDA). (1992). Development of New Stereoisomeric Drugs (Policy Statement). Federal Register, 57(88), 22249–22250.
Food and Drug Administration (FDA). (2018). Framework for FDA’s Real-World Evidence Program.
International Council for Harmonisation (ICH). (2003). Q1E: Evaluation of Stability Data.
International Council for Harmonisation (ICH). (2005). E2E: Pharmacovigilance Planning.
LaPlante, S. R., Edwards, P. J., Fader, L. D., Jakalian, A., & Hucke, O. (2011). Revealing atropisomer axial chirality in drug discovery. ChemMedChem, 6(3), 505–513.
McBride, W. G. (1961). Thalidomide and congenital abnormalities. The Lancet, 278(7216), 1358.
Rajkomar, A., Dean, J., & Kohane, I. (2019). Machine learning in medicine. New England Journal of Medicine, 380(14), 1347–1358.
Zhou, S. F. (2011). Drugs behave as substrates, inhibitors and inducers of human cytochrome P450 3A4. Current Drug Metabolism, 9(4), 310–322.