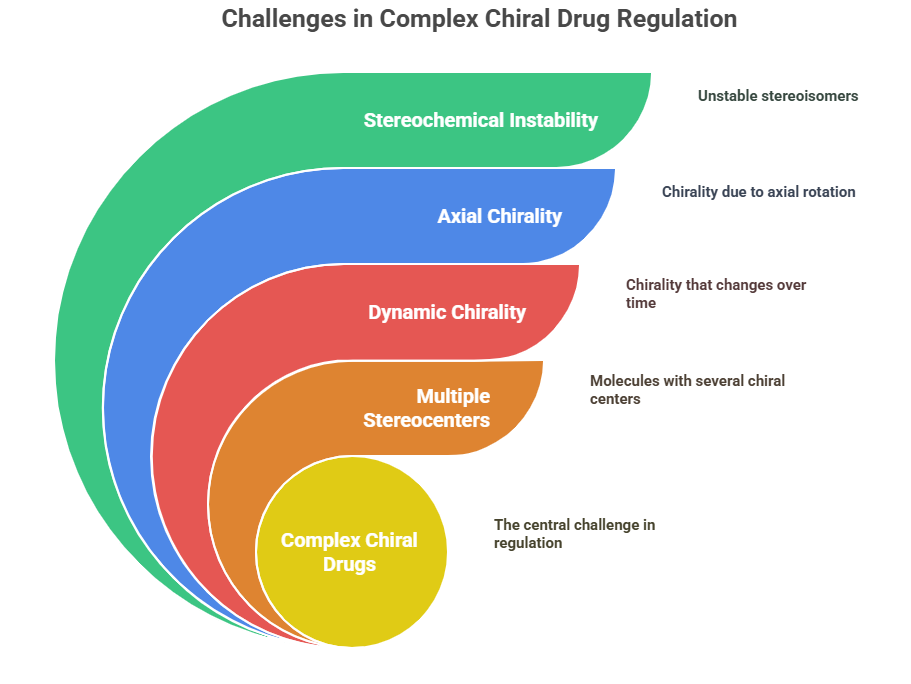
“As the molecular world grows more intricate, so do the challenges in regulating and understanding chirality.”
Introduction
While the 1990s and 2000s solidified regulatory frameworks for simple chiral drugs -classic single stereocenter, stable enantiomers –science has since moved into more complex territory. Today, pharmaceutical innovation increasingly deals with molecules featuring multiple stereocenters, dynamic chirality, axial chirality (atropisomerism), and stereochemical instability. These new realities stretch the existing regulatory and scientific paradigms developed for simpler chiral systems. In this episode, we explore the emerging challenges in characterizing, regulating, and ensuring the safety of complex chiral drugs.
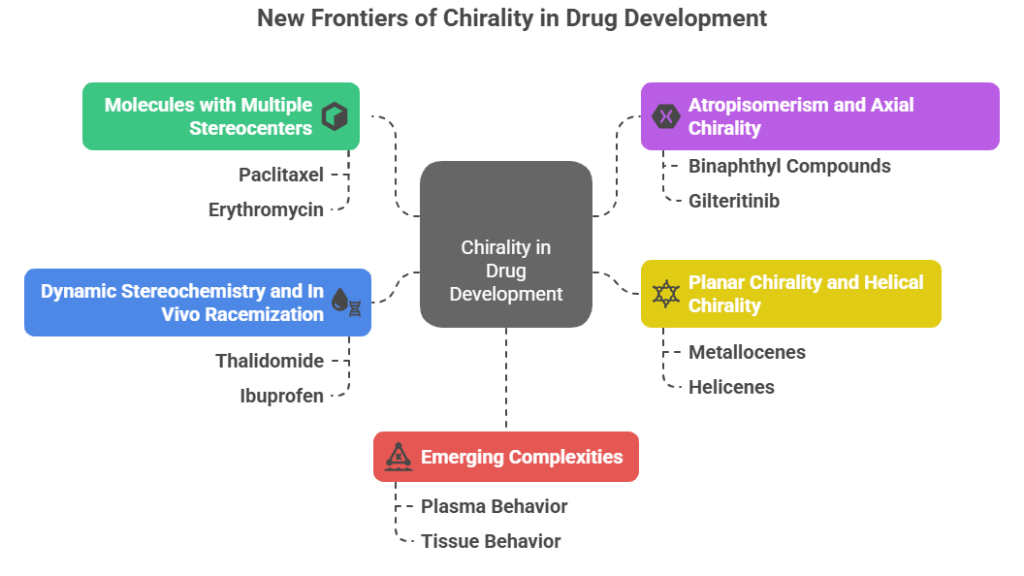
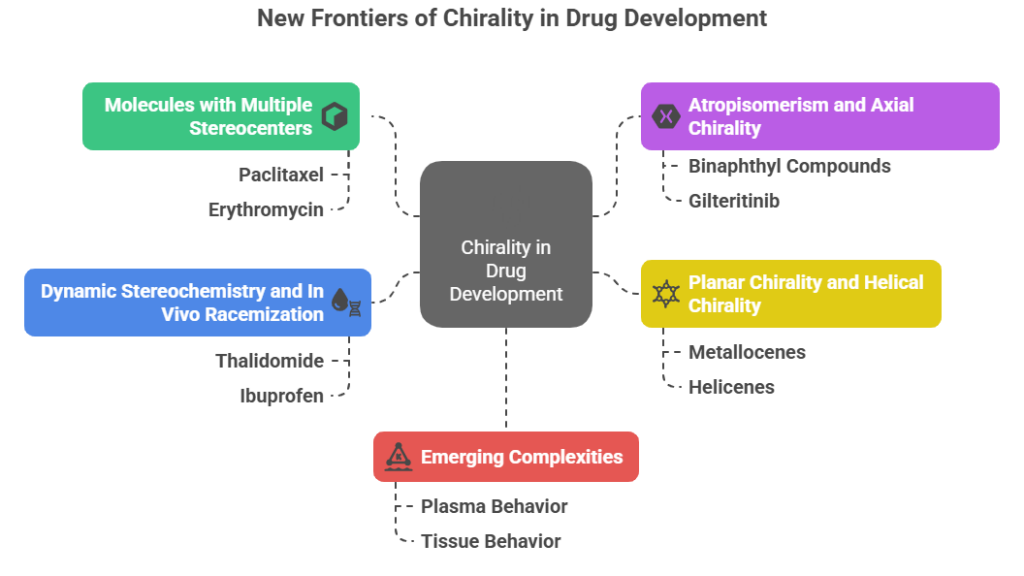
The New Frontiers of Chirality
1. Molecules with Multiple Stereocenters
Many modern small molecules -especially natural product-derived or highly functionalized drugs -possess two or more chiral centers.
Examples:
- Paclitaxel (anticancer drug) has 11 chiral centers.
- Erythromycin (macrolide antibiotic) has multiple centers critical to its biological activity.
Implication:
- Each additional stereocenter doubles the number of possible stereoisomers (2ⁿ, where n = number of centers).
- Complexity in synthesis, separation, characterization, and regulatory documentation increases exponentially.
Regulators expectation:
- Detailed stereochemical mapping of each center.
- Clear assignment of relative and absolute configuration (EMA, 1994; FDA, 1992).
- Comprehensive evaluation of all possible stereoisomeric impurities.
2. Atropisomerism and Axial Chirality
Atropisomers arise when hindered rotation around a bond leads to stable, isolable stereoisomers.
Examples:
- Binaphthyl compounds (used in chiral catalysts).
- Some kinase inhibitors (e.g., gilteritinib for leukemia) display axial chirality.
Challenges:
- Atropisomers can interconvert over time, depending on temperature and environment (LaPlante et al., 2011).
- Regulatory concern: Dynamic changes in enantiomeric purity during storage or administration.
Regulatory expectation:
- Stability studies must assess atropisomer interconversion.
- Specifications must control for changes in chiral identity over time (ICH, 1999).
3. Planar Chirality and Helical Chirality
In some complex molecular systems:
- Chirality arises from planar arrangements (e.g., metallocenes) or helical conformations (e.g., helicenes).
Though rarer in drugs currently, as medicinal chemistry expands into novel chemical space, regulators will need guidance on:
- Assigning and verifying chiral identity.
- Measuring chiral purity.
- Assessing biological implications of chirality beyond classic point chirality.
4. Dynamic Stereochemistry and In Vivo Racemization
Even classical enantiomers can undergo interconversion under physiological conditions.
Examples:
- Thalidomide racemizes rapidly in vivo, negating efforts to market safe single-enantiomer versions (McBride, 1961).
Ibuprofen undergoes partial inversion from R- to S-form after administration (Lee & Jamali, 2006).
5. Emerging complexities:
- Some modern drugs exhibit environment-dependent dynamic stereochemistry, requiring context-specific evaluation (e.g., differing behavior in plasma vs. tissues).
Regulatory expectations:
- In-depth in vivo stereochemical stability studies.
- Contextual pharmacokinetic profiling of each stereochemical species separately.
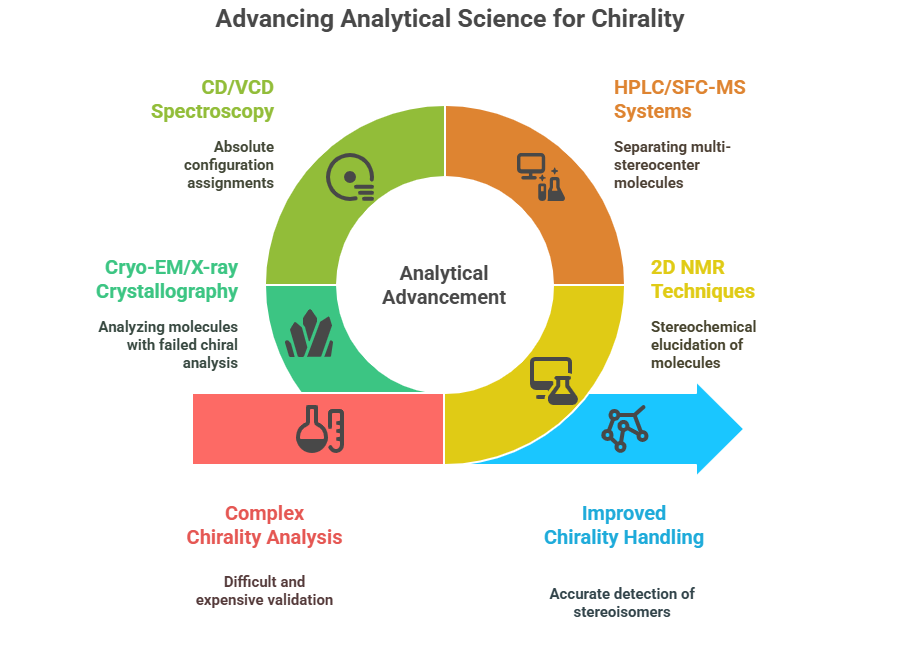
Analytical Challenges for Complex Chirality
To handle complex chirality, analytical science must keep pace.
Current advances include:
- 2D NMR techniques for full stereochemical elucidation.
- Advanced chiral HPLC/SFC-MS systems capable of separating multi-stereocenter molecules.
- Circular Dichroism (CD) and Vibrational Circular Dichroism (VCD) spectroscopy for absolute configuration assignments.
- Cryo-EM and X-ray crystallography for molecules where traditional chiral analysis fails.
Yet, challenges remain:
- Validation of chiral methods is more difficult and expensive for complex molecules.
- Minor stereoisomeric impurities may escape detection, complicating risk assessments.
Regulatory Gaps and Emerging Needs
Current regulatory frameworks (e.g., FDA 1992, EMA 1994) provide strong foundations for simple chiral drugs but leave ambiguities for complex systems.
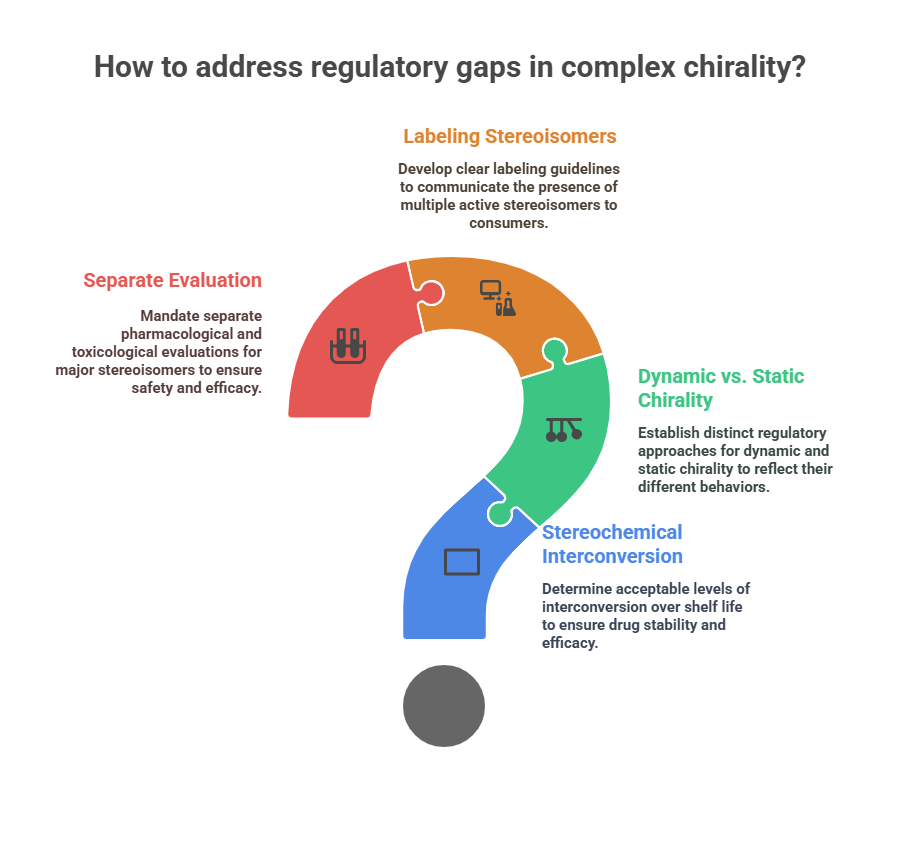
Unresolved regulatory questions include:
- How much stereochemical interconversion is acceptable over shelf life?
- Should “dynamic chirality” be treated differently from “static chirality” in specifications?
- How to label and communicate the presence of multiple active stereoisomers?
- Should separate pharmacological and toxicological evaluation be mandated for all major stereoisomers?
Agencies like the FDA and EMA are beginning to issue case-specific guidance -but comprehensive frameworks for complex chirality are still evolving.
Industry Strategies: Navigating the Complexity
Pharmaceutical companies are adopting several strategies to deal with complex chirality:
- Stereoselective synthesis: Designing pathways that favor a single stereoisomer or stereoisomeric mixture of known composition.
- Dynamic kinetic resolution: Combining racemization and selective separation to “harvest” the desired stereoisomer.
- Chiral pool synthesis: Using naturally occurring chiral building blocks (e.g., amino acids, sugars) to control stereochemistry.
From a regulatory standpoint:
- Companies must justify their strategy in regulatory submissions.
- Risk-based approaches are acceptable but must be backed by robust data.
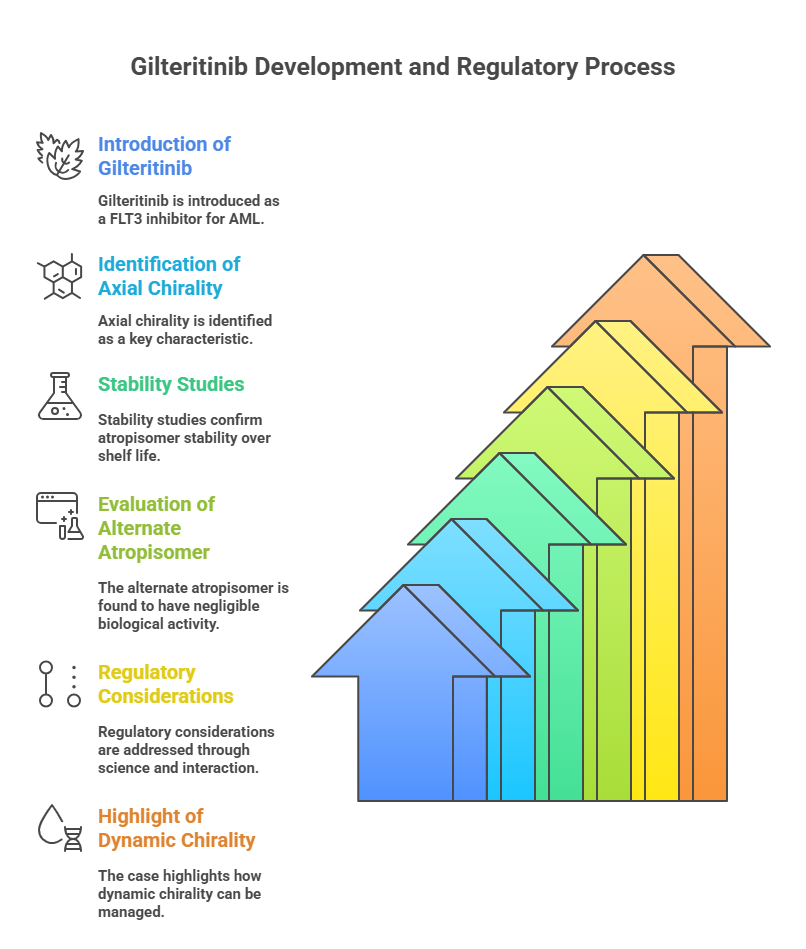
Case Study: Gilteritinib (Xospata®)
Gilteritinib, a FLT3 inhibitor for acute myeloid leukemia, exhibits axial chirality.
Regulatory considerations:
- Stability studies demonstrated atropisomer stability over the product’s shelf life.
- Separate evaluation showed that the alternate atropisomer had negligible biological activity.
This case highlights:
- How dynamic chirality can be addressed through rigorous science and thoughtful regulatory interaction.
The Future: Artificial Intelligence and Predictive Modeling
Emerging technologies may soon assist in managing complex chirality:
- AI-driven stereochemical prediction: Machine learning models to predict stability and activity of stereoisomers.
- In silico modeling of stereoselective binding and metabolism.
- Automated chiral separation design using predictive algorithms.
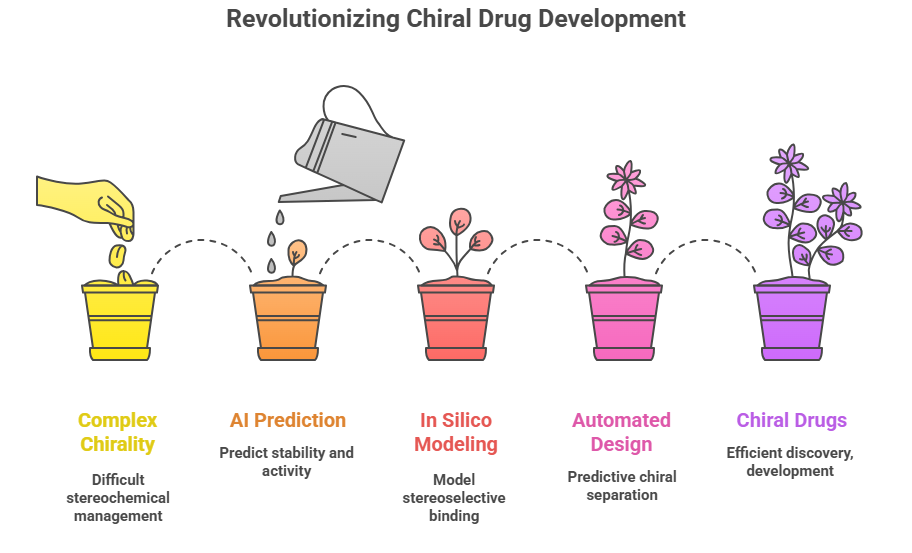
These tools could revolutionize how complex chiral drugs are discovered, developed, and regulated.
Conclusion
As medicinal chemistry ventures into increasingly complex molecular architectures, chirality will continue to challenge both scientists and regulators. Moving beyond simple left- and right-handedness, we now enter a world where chirality is fluid, multidimensional, and dynamic. Future regulatory frameworks must embrace this complexity without compromising on safety, efficacy, or quality -ensuring that patients benefit from innovation without being exposed to unseen risks.
In the final episode of this series, we will explore how post-marketing surveillance and pharmacovigilance specifically address chiral drugs -ensuring that even after approval, the mirror images continue to behave as intended.
What is in the next episode?
“Approval is only the beginning. Monitoring the mirror images over time is just as crucial. Join us for Episode 10: Future Reflections: How the Story of Chiral Drug Regulation Continues.“
References
Agranat, I., Caner, H., & Caldwell, J. (2002). Putting chirality to work: The strategy of chiral switches. Nature Reviews Drug Discovery, 1(10), 753–768.
European Medicines Agency (EMA). (1994). Investigation of Chiral Active Substances (CPMP Note for Guidance 3CC29a).
Food and Drug Administration (FDA). (1992). Development of New Stereoisomeric Drugs (Policy Statement). Federal Register, 57(88), 22249–22250.
International Council for Harmonisation (ICH). (1999). Q6A: Specifications – Test Procedures and Acceptance Criteria for New Drug Substances and New Drug Products: Chemical Substances.
LaPlante, S. R., Edwards, P. J., Fader, L. D., Jakalian, A., & Hucke, O. (2011). Revealing atropisomer axial chirality in drug discovery. ChemMedChem, 6(3), 505–513.
Lee, E. J., & Jamali, F. (2006). Clinical pharmacokinetics of ibuprofen. Clinical Pharmacokinetics, 6(5), 402–415.
McBride, W. G. (1961). Thalidomide and congenital abnormalities. The Lancet, 278(7216), 1358.
Zhou, S. F. (2011). Drugs behave as substrates, inhibitors and inducers of human cytochrome P450 3A4. Current Drug Metabolism, 9(4), 310–322.