Beta blockers, also spelled β-blockers, used as antihypertensive agents (lowering blood pressure), represent a class of chiral drugs that are marketed as racemates since the distomer exhibits no undesirable adverse effects. Three most important β-blockers viz. propranolol, atenolol, and metoprolol are marketed as racemic mixture.
Chirality and biological activity
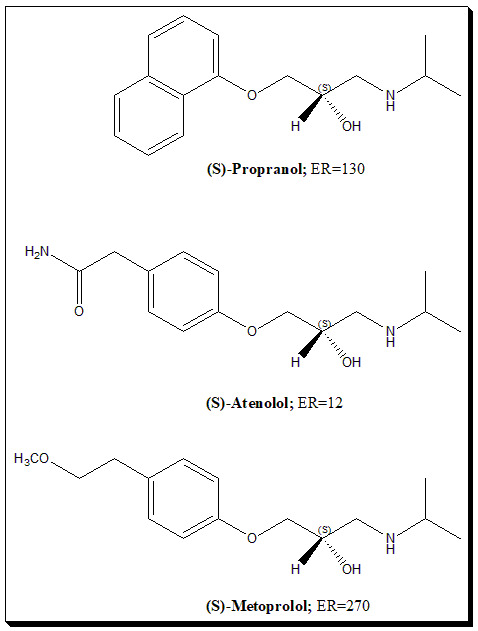
β-blockers are aryloxy propanolamines with one stereogenic center and exists as a pair of enantiomers. It is observed that their therapeutic effect resides entirely in the (S)-enantiomer (eutomer). This form holds a strong structural resemblance to the adrenergic hormone noradrenaline (ER = 300). (S)-propranolol is found to be 130 times as active as its (R)-enantiomer (eudisimic ratio, ER =130), reflecting that the (R)-enantiomer is totally inactive (distomer). Timolol (ER=12) and Metoprolol (ER =270).
Note: The observed low values of ER for some of the β-blockers may be due to traces of eutomer in the sample. ER is eudisimic ratio and is eutomer/distomer ratio and reflects the degree of enantioselectivity. To know more about eutomer, distomer and eudisimic ratio nomenclature read @ <https://chiralpedia.com/blog/chiral-twins-identical-but-not-really/>
The decision to market these drugs as racemates probably would have been due to fact that the distomer is inert and has no serious side-effects. And also could be the lack of chiral technology available then to separate them. Had the β-blockers be introduced today as new drugs they would have almost certainly be marketed as unichiral drugs. This so because even if the distomer is inert, isomeric ballast, it will add onto the metabolic load of the patient.
Therapeutic category
Anti-hypertensive agents
Nomenclature
1-naphthalen-1-yloxy-3-(propan-2-ylamino)propan-2-ol
Exercises
Understand the nomenclature system, stereochemical terms viz. eutomer, distomer, eudisimic ratio
References
Roger A, Sheldon, Chirotechnology: industrial synthesis of optically active compounds, Marcel Dekker,1993.
Chiral drugs. Wikipedia, Wikipedia Foundation, 09/10/2022. https://en.wikipedia.org/wiki/Chiral_drugs and references therein
Ariëns, Everardus J. (1986). “Stereochemistry: A source of problems in medicinal chemistry”. Medicinal Research Reviews. 6 (4): 451–466. doi:10.1002/med.2610060404
Ariëns, E. J. (1984). “Stereochemistry, a basis for sophisticated nonsense in pharmacokinetics and clinical pharmacology”. European Journal of Clinical Pharmacology. 26 (6): 663–668. doi:10.1007/BF00541922