Lead
Chiral resolution has been an essential process in various industries, particularly pharmaceuticals, where separating enantiomers is critical for drug efficacy and safety. Traditional techniques such as chromatography, crystallization, and kinetic resolution have served as the backbone of chiral separation. However, these methods can be time-consuming, costly, and inefficient, especially at large scales. As industries evolve and demand for enantiopure compounds increases, there is a growing need for innovative and efficient methods of chiral resolution. Enter unconventional approaches that leverage cutting-edge technologies like electroassisted separation, wireless methods, and the chiral-induced spin selectivity (CISS) effect. These emerging methods hold the potential to revolutionize the field by offering faster, more sustainable, and less resource-intensive solutions.
1. Why New Approaches Are Needed in Chiral Resolution
The demand for chiral compounds, particularly in the pharmaceutical, agrochemical, and fine chemical industries, has grown exponentially over the years. Traditional methods like chromatography and crystallization, though effective, come with limitations such as high energy consumption, excessive use of solvents, and lengthy processing times. These challenges have driven the search for more efficient and sustainable alternatives. Additionally, as regulatory bodies such as the U.S. Food and Drug Administration (FDA) impose stricter guidelines on the purity of enantiomeric drugs, the need for faster and more precise chiral resolution techniques has become paramount.
Innovations in chiral separation techniques are highlighted. A comparative review is presented schematically.
There is a growing interest in methods that can be scaled easily for industrial applications while maintaining the precision required for enantiomer separation. Traditional techniques often struggle with scalability, leading to inefficiencies in high-throughput settings. New approaches, such as electro-assisted separation and wireless electrochemistry, provide an opportunity to overcome these hurdles. By leveraging external stimuli like electric and magnetic fields, these methods can offer a higher degree of control over the separation process, reducing the need for solvents and lowering energy consumption (Pinto, 2020).
2. Overview of Emerging Techniques
Several unconventional methods are gaining attention in the field of chiral resolution. Among the most promising are electro-assisted methods, which use electric fields to enhance the separation of enantiomers. These techniques offer a significant improvement over traditional methods in terms of efficiency and precision. Another innovative approach is wireless electro-assisted chiral resolution, which relies on bipolar electrochemistry to separate enantiomers without the need for direct electrical connections, providing more flexibility in the separation process.
Unconventional Enantioseparation
Physical methods
Typically, separation methods work by exploiting the different physical properties of the components in a mixture. However, since the two enantiomers of a molecule share the same scalar properties, we need a chiral environment to separate them. While traditional physical methods like adsorption, filtration, and crystallization are well-known and widely used, the innovative aspect lies in employing advanced chiral selectors or external stimuli to achieve enantioseparation.
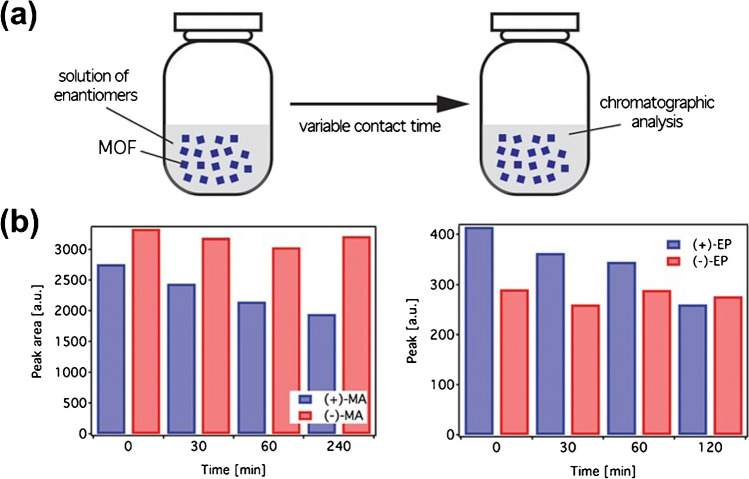
Selective adsorption of chiral molecules
Selective adsorption of chiral molecules on designed selectors, using intermolecular interactions or host cavity specificity, requires only physical mixing for a specific time (Fig. 1a). Homochiral MOFs, due to their structural properties, are ideal for this. For instance, a carboxylated MOF achieved 99% enantiomeric excess for (S)-1-(1-naphthyl) ethanol. Isostructural chiral MOFs effectively adsorbed chiral amines with high enantioselectivity (>80%). In this work, a chiral Cu(II) 3D MOF based on gly-L-Hys-Gly facilitated time-dependent enantioselective separation of (+)-EP and (+)-MA from a racemic mixture through host-guest interactions (Fig. 1b). However, industrial scaling is limited by high costs and time-consuming procedures.
Electro-assisted Methods
How Electric Fields Enhance Enantioselective Separation
Electroassisted methods leverage electric fields to enhance the separation of enantiomers by inducing selective interactions between chiral molecules and charged surfaces. In these processes, the electric field influences the movement of charged species, allowing for the selective migration of one enantiomer over the other. This can be achieved by introducing chiral selectors into the system that interact differently with the enantiomers under the influence of an electric field (Sui, 2023).
One of the primary advantages of electroassisted separation is its ability to operate under mild conditions, reducing the need for harsh solvents and high temperatures. This makes it an attractive option for sensitive compounds, such as those used in pharmaceutical formulations. Additionally, the precision afforded by electric fields allows for a higher degree of control over the separation process, resulting in greater purity and higher yields compared to traditional methods.
The mechanism behind electro-assisted methods is relatively straightforward: an electric field is applied across a medium containing a racemic mixture. The chiral molecules respond to the electric field differently based on their charge distribution and molecular structure. By adjusting the strength and orientation of the electric field, it is possible to selectively move one enantiomer toward the anode or cathode, leaving the other enantiomer behind. This selective migration enhances the separation efficiency and reduces processing time, making electro-assisted methods highly scalable for industrial applications.
Applications in Small-Scale and Industrial Processes
Electroassisted chiral separation methods have found applications in both small-scale laboratory settings and large-scale industrial processes. In small-scale applications, these methods are particularly useful for the development of pharmaceutical compounds, where precision and purity are paramount. The ability to fine-tune the separation process using electric fields allows researchers to achieve enantiomeric purity levels that are difficult to obtain using conventional techniques. This is especially important in the development of new drugs, where even small amounts of the wrong enantiomer can lead to significant differences in therapeutic outcomes.
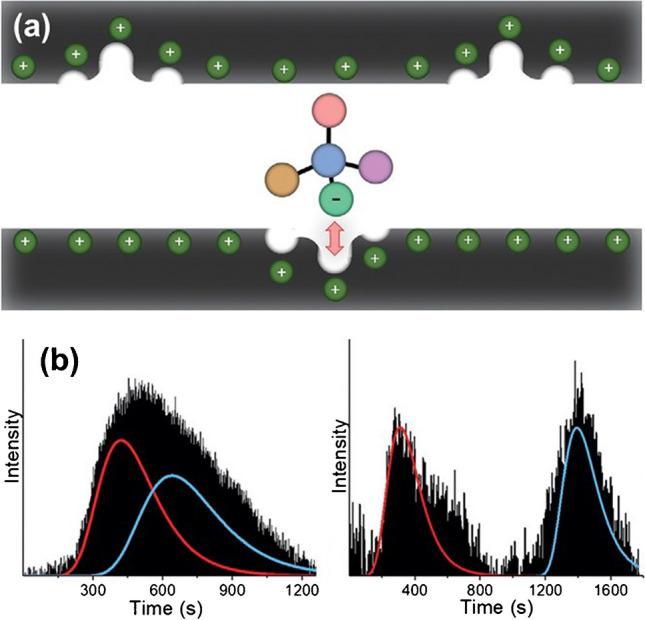
Electroassisted Chiral Separation Approach
An electroassisted approach uses an electric field to trigger the adsorption or desorption of a chiral analyte. This process induces electrostatic interactions between charged analytes and the polarized surface, with the electrode acting as the stationary phase (Fig. 4a). By encoding chiral information on the electrode surface, it’s possible to leverage the synergy between electrostatic interactions and chiral cavities. Enantioenriched mixtures were injected into a microchannel decorated with a chiral-encoded porous platinum film by fine-tuning the applied electric field (Fig.4b).
At the industrial level, electroassisted methods offer the potential for continuous processing, making them ideal for high-throughput production environments. By incorporating these methods into existing production lines, manufacturers can reduce the need for expensive and time-consuming purification steps. Additionally, the use of electric fields allows for greater control over the separation process, enabling manufacturers to optimize their operations for different compounds without the need for extensive retooling. A chiral-imprinted mesoporous platinum surface has been successfully employed for the electroassisted enantioseparation of the two enantiomers of tryptophan (Tryp) and tyrosine.
One of the most promising industrial applications of electro-assisted separation is in the production of chiral intermediates for pharmaceuticals. These intermediates are often difficult to separate using traditional methods due to their complex molecular structures. By using electro-assisted techniques, manufacturers can achieve higher yields and greater purity, reducing the overall cost of production while maintaining the quality of the final product (Sui, 2023).
Case Study: Electro-assisted Enantioselective Crystallization
A notable application of electroassisted methods is in enantioselective crystallization, where electric fields are used to influence the formation of crystals that contain predominantly one enantiomer. This process has been applied in the separation of complex pharmaceutical compounds, where traditional crystallization methods have proven inefficient. In one case, electroassisted crystallization was employed to resolve a racemic mixture of a pharmaceutical intermediate, leading to significantly higher purity and yield compared to conventional crystallization techniques.
The process works by applying an electric field to a solution containing the racemic mixture. As the solution cools, crystals begin to form, but the presence of the electric field influences the orientation and packing of the molecules, favoring the formation of one enantiomer over the other. This selective crystallization process can be fine-tuned by adjusting the strength and orientation of the electric field, allowing for greater control over the purity of the final product.
This method has proven particularly useful for compounds that are difficult to resolve using traditional methods, such as those with similar physical properties between enantiomers. The use of electric fields provides an additional layer of control, enabling more efficient and effective separation.
Wireless Electro-assisted Chiral Resolution
Introduction to Bipolar Electrochemistry
Bipolar electrochemistry is an innovative method that utilizes wireless electrochemical systems to achieve chiral resolution without the need for direct electrical connections. In a bipolar electrochemical setup, an external electric field is applied to a conductive object, known as a bipolar electrode (BPE), creating a polarization gradient across the electrode. This polarization induces a redox reaction at opposite ends of the BPE, allowing for the selective separation of enantiomers).
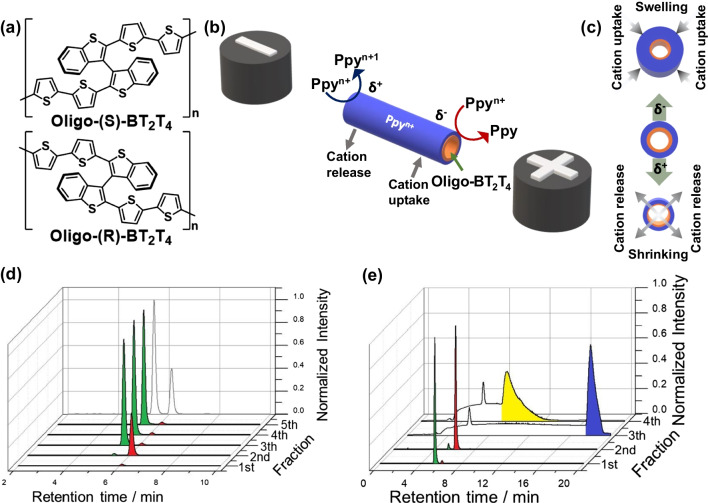
Wireless electroassisted approach
a) Chemical structures of enantiopure oligo-(S)- and oligo-(R)-BT2T4. b) Illustration of the wireless enantioselective loading/pumping mechanism, showing asymmetric polarization, electrochemical reactions, and cation exchange. c) Schematic of electric field-induced asymmetric swelling and shrinking process, representing cation exchange. d) Chromatograms of carvone enantioenriched mixture (S:R) 70:30 from chiral tube with (S)-oligomer. e) Chromatograms of carvone and N, N-dimethyl-1-ferrocenylethylamine racemates from chiral tube with (R)-oligomer. Green/red indicate (S)/(R)-carvone; yellow/blue indicate (S)/(R)-N, N-dimethyl-1-ferrocenylethylamine.
Unlike traditional electrochemical methods, which require direct electrical contact with the electrode, bipolar electrochemistry relies on the wireless nature of the system, making it highly versatile and adaptable for a variety of applications. This technique is particularly useful in environments where direct contact with the electrode is impractical, such as in microfluidic systems or in cases where multiple reactions need to be carried out simultaneously.
The principle behind bipolar electrochemistry is simple but effective: when an electric field is applied to a BPE, the electrode becomes polarized, creating a potential difference between its two ends. This potential difference drives redox reactions that can be tailored to selectively oxidize or reduce one enantiomer over the other. By carefully controlling the applied field, it is possible to achieve highly selective enantioselective separation without the need for complex setups or additional reagents.
How Wireless Methods Can Enhance Separation Processes
Wireless electroassisted methods offer several advantages over traditional electrochemical techniques. First, the lack of direct electrical connections makes the system more flexible and easier to integrate into existing production lines. This is particularly important in industrial settings, where scalability and adaptability are key concerns. By using wireless methods, manufacturers can achieve high-throughput chiral resolution without the need for extensive reconfiguration of their processes.
Second, the wireless nature of bipolar electrochemistry allows for more precise control over the separation process. Because the system is not constrained by direct electrical connections, it is possible to fine-tune the polarization of the BPE to achieve optimal separation conditions. This level of control is particularly useful for complex compounds that require highly specific separation parameters.
Finally, wireless methods are more environmentally friendly compared to traditional electrochemical techniques. By eliminating the need for direct electrical connections and reducing the amount of reagent required for separation, these methods reduce waste and energy consumption, making them an attractive option for industries looking to adopt more sustainable practices (Pinto, 2020).
Case Study: Wireless Enantioselective Electro-Pumping
One innovative application of wireless electroassisted chiral resolution is enantioselective electro-pumping, a process that uses the wireless nature of bipolar electrochemistry to selectively move one enantiomer through a microfluidic system. This technique has been successfully applied in the separation of amino acids, where the polarization of a BPE induced selective movement of the desired enantiomer, effectively “pumping” it through the system while leaving the undesired enantiomer behind.
The key to this process lies in the careful control of the electric field applied to the BPE. By adjusting the strength and orientation of the field, researchers were able to achieve highly selective separation of enantiomers with minimal energy input. This method has proven particularly useful in microfluidic systems, where traditional separation techniques are often impractical due to the small volumes involved.
The Chiral-Induced Spin Selectivity (CISS) Effect
Overview of the CISS Phenomenon
The Chiral-Induced Spin Selectivity (CISS) effect is a groundbreaking discovery in chiral resolution, enabling enantiomer separation based on their interaction with electron spins. This additive-free approach offers a green chemistry solution for chiral separation, showing great potential for industrial applications. Emerging techniques leveraging the CISS effect could revolutionize chiral separation by selecting one spin orientation over another, enhancing transport properties and efficiency.
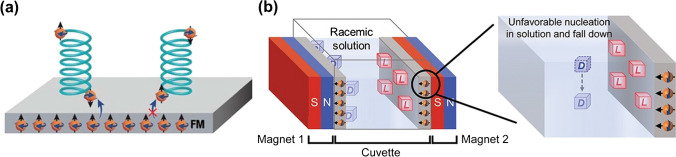
Proposed recognition mechanism based on the chiral-induced spin selectivity
The chiral-induced spin selectivity (CISS) phenomenon has enabled an additive-free crystallization method. This approach leverages the coupling of the polarized spin of a chiral molecule with the electron spin of a ferromagnetic material under an orthogonal magnetic field (Fig. a). Enantioselective crystallization of asparagine, glutamic acid, and threonine was achieved via spin alignment between the chiral molecule and the ferromagnetic surface. Altering the external magnetic field orientation caused a symmetry break, allowing selective resolution of stereoisomers. Recently, this spin effect was used for simultaneous resolution of conglomerates. Fine-tuning the spatial distribution of two ferromagnetic surfaces with opposite magnetization allowed spin-selective crystallization of different enantiomers on each surface (Fig. b). However, scaling this approach to industrial levels faces challenges due to the complexity of synthesizing enantioselective polymers and the large size of the magnets/electromagnets required.
The CISS effect has generated significant interest because it offers an entirely new approach to chiral resolution that is both highly efficient and environmentally friendly. Unlike traditional methods that rely on chemical reagents or complex setups, the CISS effect exploits the intrinsic properties of chiral molecules and their interactions with electron spins. This additive-free approach has the potential to reduce both the cost and environmental impact of chiral resolution, making it an attractive option for industries looking to adopt greener practices.
Applications in Additive-Free Enantioselective Crystallization
One of the most promising applications of the CISS effect is in additive-free enantioselective crystallization. This process exploits the spin-selective interactions between chiral molecules and electron spins to achieve high-purity separation without the need for additional reagents. By aligning the electron spins in a magnetic field, researchers can influence the crystallization process to favor the formation of one enantiomer over the other, leading to highly efficient separation.
The use of the CISS effect in crystallization is particularly appealing for industries focused on sustainability, as it eliminates the need for chemical additives that can generate waste and require additional purification steps. In a recent study, the CISS effect was successfully applied to the crystallization of a pharmaceutical intermediate, achieving enantiomeric purities of over 99% without the need for external chiral selectors. This represents a significant breakthrough in the field of chiral resolution, offering a more efficient and environmentally friendly alternative to traditional crystallization methods.
Case Study: Spin-Selective Crystallization of Amino Acids
A notable case study involving the CISS effect is the spin-selective crystallization of amino acids, a process that leverages the interaction between electron spins and chiral molecules to achieve enantioselective separation. In this study, researchers applied a magnetic field to a solution containing a racemic mixture of amino acids, aligning the electron spins and influencing the crystallization process to selectively favor one enantiomer.
Challenges and the Road Ahead
Current Limitations in Scaling Up These Unconventional Methods
While the potential of unconventional methods such as electroassisted separation, wireless electrochemistry, and the CISS effect is undeniable, several challenges remain in scaling up these techniques for industrial use. One of the primary limitations is the complexity of the setups required for these methods. For example, the precise control of electric fields and magnetic fields needed for electroassisted and CISS-based methods can be difficult to achieve on a large scale, especially in continuous production environments.
Additionally, the cost of developing and maintaining the specialized equipment required for these methods may be prohibitive for some industries, particularly those with tight margins. While the long-term benefits of these methods in terms of efficiency and sustainability are clear, the initial investment in infrastructure may slow their adoption in the short term (Sui, 2023).
Future Prospects for Industrial Applications
Despite these challenges, the future prospects for industrial applications of unconventional chiral resolution methods are bright. As research continues to refine these techniques and develop more cost-effective solutions, it is likely that industries will begin to adopt these methods on a larger scale. Advances in materials science, particularly in the development of more efficient and cost-effective electrodes and magnetic systems, will play a key role in making these methods more accessible.
Additionally, as industries continue to prioritize sustainability and green chemistry initiatives, the additive-free nature of the CISS effect and the low-energy requirements of wireless electroassisted methods will become increasingly attractive. Over time, these unconventional methods could become the standard for chiral resolution, particularly in industries that require high-purity enantiomers at scale.
Conclusion
The Role of Unconventional Methods in the Future of Chiral Resolution
Unconventional methods such as electroassisted separation, wireless electrochemistry, and the CISS effect are poised to play a significant role in the future of chiral resolution. These techniques offer numerous advantages over traditional methods, including greater efficiency, scalability, and environmental sustainability. While challenges remain in terms of scaling up these methods for industrial use, ongoing research and technological advancements are likely to overcome these hurdles in the near future.
As industries continue to demand more efficient and sustainable solutions for chiral resolution, unconventional methods will become increasingly important. Their ability to achieve high enantiomeric purity with minimal waste and energy consumption makes them ideal for applications in pharmaceuticals, agrochemicals, and fine chemicals. As these techniques mature and become more widely adopted, they have the potential to revolutionize the field of chiral resolution, providing faster, cleaner, and more efficient solutions for separating enantiomers (Sui, 2023).
Further Reading
Hangh, X. (2024). Advancement of Chiral Resolution and Separations: Techniques and Applications. Highlights in Science, Engineering and Technology, 83, pp.305-310.
Malacarne F, Grecchi S, Niamlaem M, Bonczak B, Salinas G, Arnaboldi S. Unconventional approaches for chiral resolution. Anal Bioanal Chem. 2024, 416 (16), 3677-3685. doi: 10.1007/s00216-024-05329-2.
Pinto, M. (2020). Chiral Separations in Preparative Scale: A Medicinal Chemist’s Viewpoint. Journal of Medicinal Chemistry, 63(14), pp.7889-7899.
Sui, H. (2023). Strategies for Chiral Separation: From Racemate to Enantiomer. Royal Society of Chemistry.
Suliman, A. (2023). Emerging Developments in Separation Techniques and Analysis of Chiral Pharmaceuticals. Molecules, 28(5), pp.1-12.