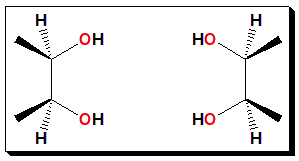
“If you immediately identified this as a molecule with an internal plane of symmetry (and hence an achiral molecule, incapable of having an enantiomer), congratulations. If not, you just fell into The Meso Trap”.
Source: https://www.masterorganicchemistry.com/2011/01/12/the-meso-trap/
The meso concept is a common aspect in examinations and tests to evaluate how good you understand the concept of chirality So let us try to understand meso-compounds with case studies.
It is suggested that the reader may first go through the earlier blog “Erythro- and Threo- prefixes: the (same-) or (opposite-) side?” for a better understanding.
It is important to realize that the 2n rule predicts only the maximum number of stereoisomers possible in compounds with more than one center of chirality. For example, some compounds with two chiral centers and having a symmetric structure may have only three stereoisomeric forms. In a constitutionally symmetrical molecule, the stereogenic/chiral centers equidistant from the geometrical center of the molecule are identically substituted.
Let us examine the stereochemistry of tartaric acid, a simple organic dicarboxylic acid, an example for n is even. It can easily be seen that both the chiral centers in its structure are identically substituted. In other words both the the chiral centers are identical. The structures are represented using Fischer projection formula (I, II, III, & III’).
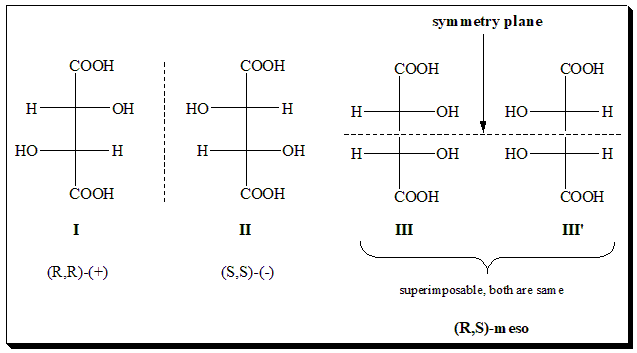
One pair of enantiomers is (I) and (II). The dextrorotatory form has the (R,R)-configuration while its mirror-image levorotatory enantiomer is (S,S). The expected second pair of enantiomers (III), (R,S) and (III’), (S,R) does not exist since they are superimposable. Hence is achiral and identical. Take note that the molecule (III’) has a horizontal plane of symmetry ( internal mirror plane indicated by dotted line) dividing the molecule into two identical parts such that one is reflection of the other.. The achiral, optically inactive stereoisomer (III) is called the meso-form, and it holds a diastereomeric relationship with the optically active stereoisomers (I and II). Thus tartaric acid exists as 2+1 stereoisomers.
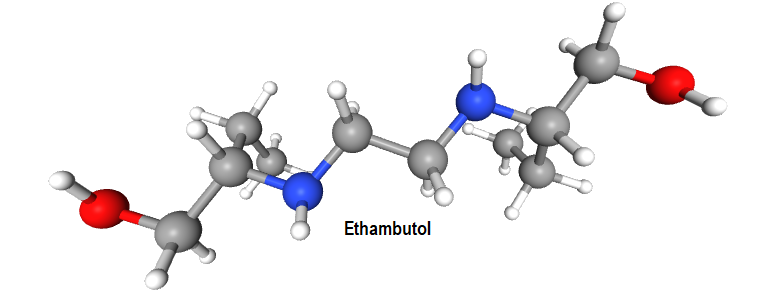
The antitubercular agent ethambutol is an example worthy of special mention and exists as 2+1 stereoisomers. It exists as one pair of enantiomers. The second pair of enantiomers does not exist since they are superimposable. It is achiral and is referred to as the meso-form. It holds a diastereomeric relationship with the optically active stereoisomers.
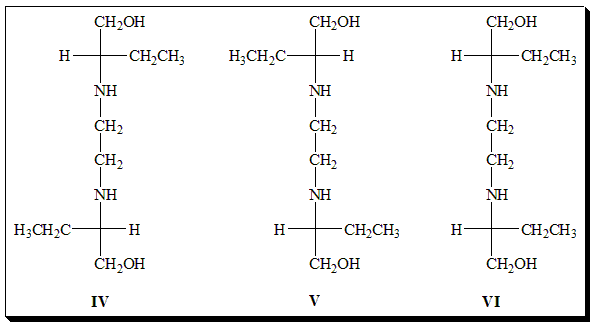
Among the stereoisomers of Ethambutol (IV, V, VI) identify the enantiomeric pair and the meso-form. Assign absolute configuration to the chiral centers. Comment on the biological activity.
Practice exercises
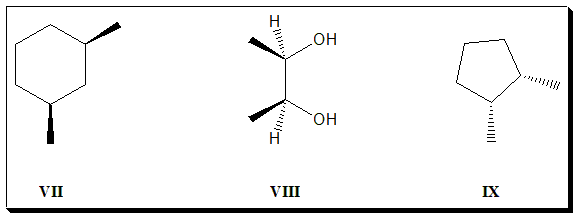
1. Do the following simple organic molecules have plane of symmetry? If so identify and label them as “chiral or “achiral”.
References
Jerry March, Advanced Organic Chemistry: Reactions, Mechanisms and Structure, John Wiley & Sons Inc., New York, 2006.
Bernard Testa, Principles of organic stereochemistry, Marcel Dekker Inc., New York, 1979.
Chiral drugs. Wikipedia, Wikipedia Foundation, 22/03/2022. https://en.wikipedia.org/wiki/Chiral_drugs

Kindly update topics such as Racemic modification and resolution of Racemic mixture, Asymmetric synthesis, Stereospecific and stereoselectice reactions. (From Karpagam College of Pharmacy, Coimbatore.)
As desired, I shall try to make posts on the topics that you are interested in.
List of sequence rule is missing.. Kindly add it
For sequence rule please read my earlier blog entitled “Naming enantiomers: the left-(or right-) handed?”.
Ok sir, thank you I will go thorough
Topic Cis trans
Difference between cis and trans please showing noted as divide into two boxs ….
Thank u so much sir
Please refer carewell pharma,it will be useful to u
Thank you for the suggestion. As you know chiralpedia.com is an open web resource for chiral science. It is not a syllabus and exam oriented website. The purpose is to promote chiral science. This site is meant for academia (faculty, research scholar, and students who are novice to the field of chiral technology) and industry as target audience. The chiralpedia.com/blogs are specifically on the basics of stereochemistry and are posted for students, who are beginners to stereochemistry. I would suggest you to take a tour of chiralpedia.com and get back to me with your feedback.
Easily understandable…. Much better if sequence rules are explained
Glad it is easily understandable. As far as the sequence rule is concerned you may read my earlier blog entitled “Naming enantiomers: the left-(or right-) handed?”.
Sure. Thank you
Very use to study
Thank u so much sir