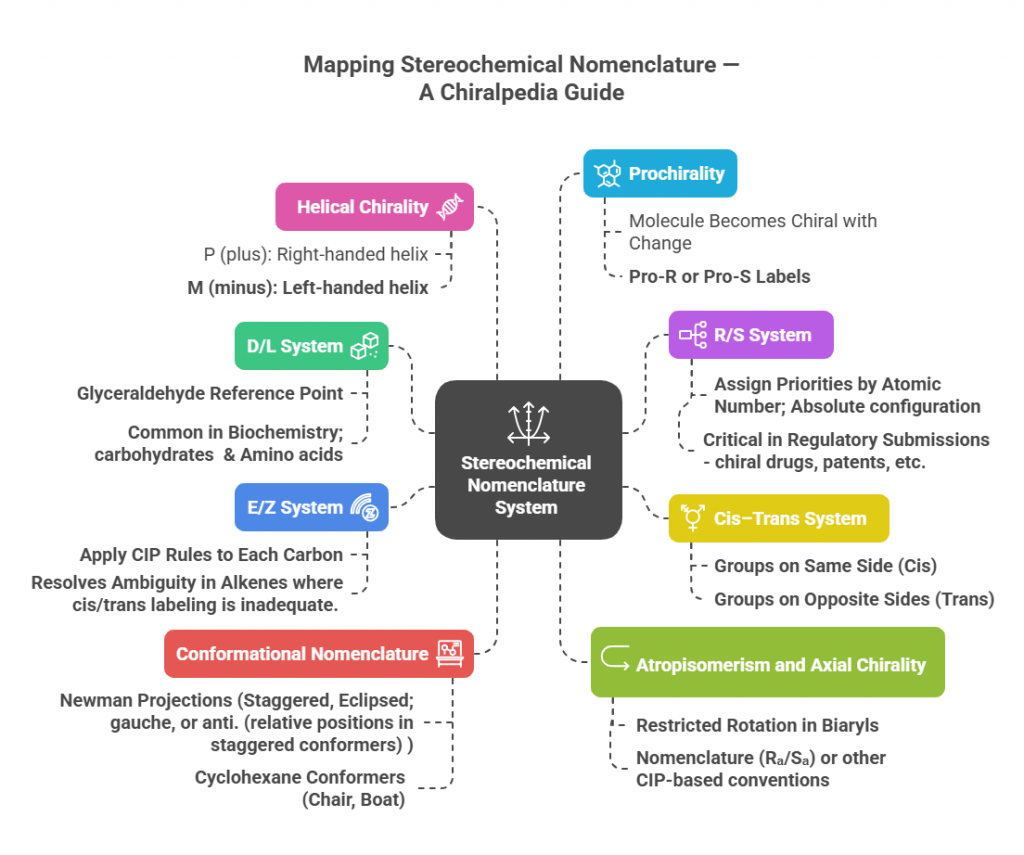
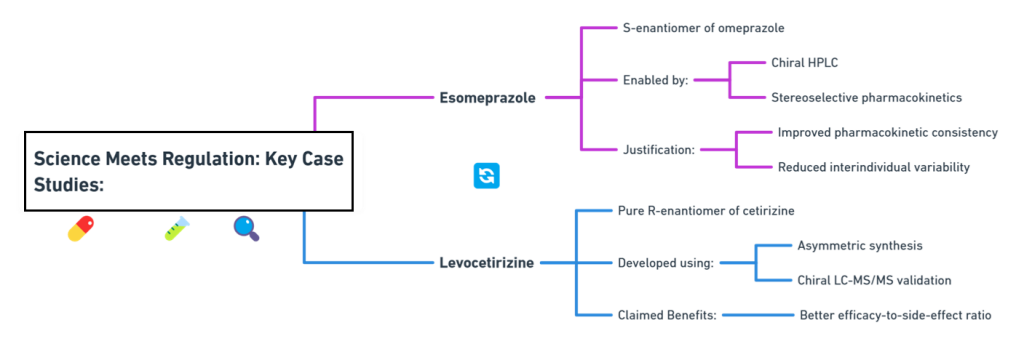
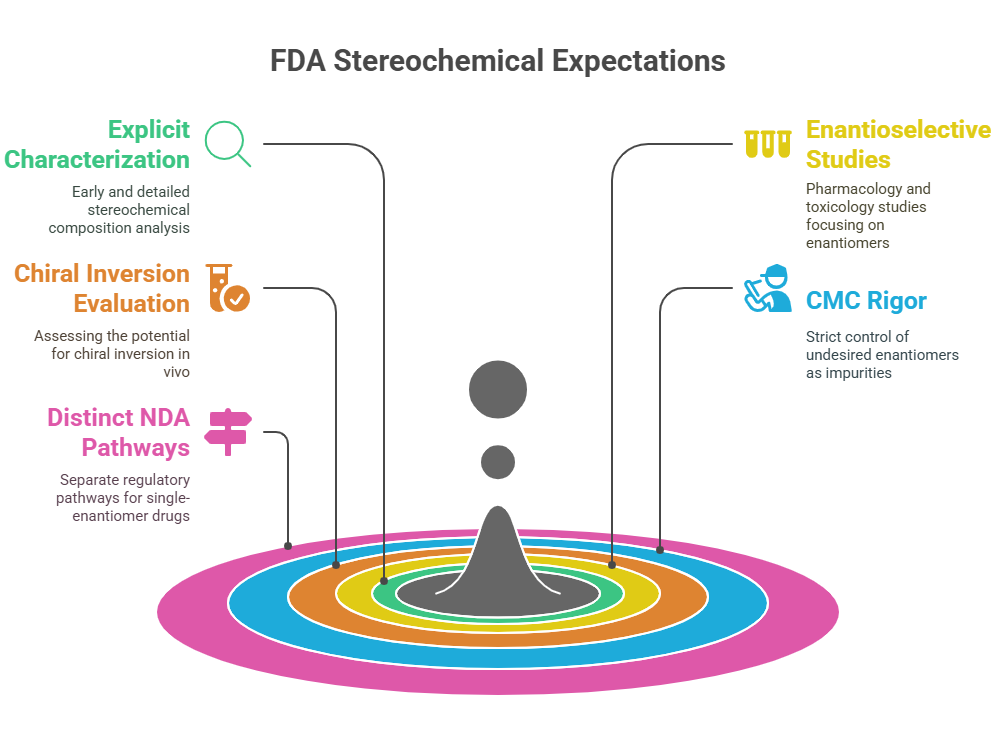
Mapping Stereochemical Nomenclature: A Chiralpedia Guide
Stereochemistry, the study of spatial arrangements of atoms in molecules, demands a precise and universally accepted nomenclature system. Unlike simple chemical formulas, which only indicate connectivity, stereochemical nomenclature conveys three-dimensional information essential for understanding molecular behavior, biological interactions, and pharmaceutical effects. Several systems have been developed to capture these subtle but critical differences. 🔬✨ Stereochemical Nomenclature System — Now in a Visual Story! Stereochemical naming systems are often tucked away in textbooks 📚, dense and …
Mapping Stereochemical Nomenclature: A Chiralpedia Guide Read More »