(S,S)-(+)-Ethambutol is powerful and selective antitubercular drug. It is a classical example of an old drug that was introduced for clinical use in its chirally pure form.
Chirality and biological activity
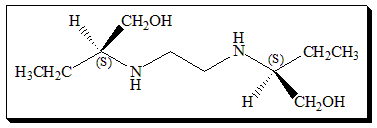
Ethambutol contains two constitutionally symmetrical stereogenic centers in its structure and exists in three stereoisomeric forms. An enantiomeric pair (S,S)- and (R,R)-ethambutol, along with the achiral stereoisomer called meso-form. The activity of the drug resides in the (S,S)-(+)-enantiomer which is 500 and 12 fold more potent than the (R,R)-ethambutol and the (R,S)-ethambutol ( meso-form) respectively. On the contrary, all the three isomers are equipotent in terms of the major side-effect of the drug, optic neuritis, that can cause blindness. It is observed that toxicity of ethambutol is related to both dose and duration of treatment. Hence the use of (S,S)-enantiomer greatly enhanced the risk/benefit ratio.
Therapeutic category
Anti-tuberculosis drug
Nomenclature
(S,S)-N,N’-Ethylenebis(2-aminobutan-1-ol)
Exercise
- Explain constitutionally symmetrical stereogenic center
- Number the ethambutol structure and relate to nomenclature
- Draw the stereochemical structures of (R,R)- and (R,S)- isomer of ethambutol and understand the stereoisomeric relationship
References
Chiral drug. Wikipedia, Wikipedia Foundation, 26/07/2022. https://en.wikipedia.org/wiki/Chiral_drugs and references therein
Shah, R.R., Improving clinical risk/benefit through stereochemistry, In Eichelbaum, Michel F., Testa, Bernard, Somogyi, Andrew (Eds.). Stereochemical aspects of drug action and disposition, Springer, Page 401-32, 2003.
Blessington Bernard, Ethambutol and tuberculosis, A neglected and confused chiral puzzle, In Hassan Y Abul-Enein and Irving W Wainer (Eds.).The impact of stereochemistry on drug development and use, New York: John Wiley & Sons, New York. pp. 235–261, 1997.
Harkishan Singh and V.K. Kapoor. Medicinal chemistry and pharmaceutical chemistry, Vallabh Prakashan, New Delhi, Page 614-15, 2012.