Episode 10: Future Reflections: How the Story of Chiral Drug Regulation Continues
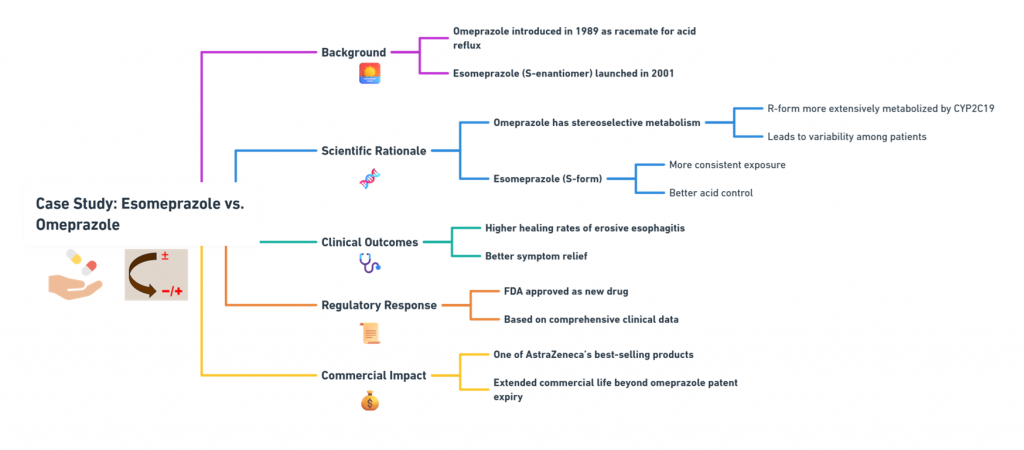
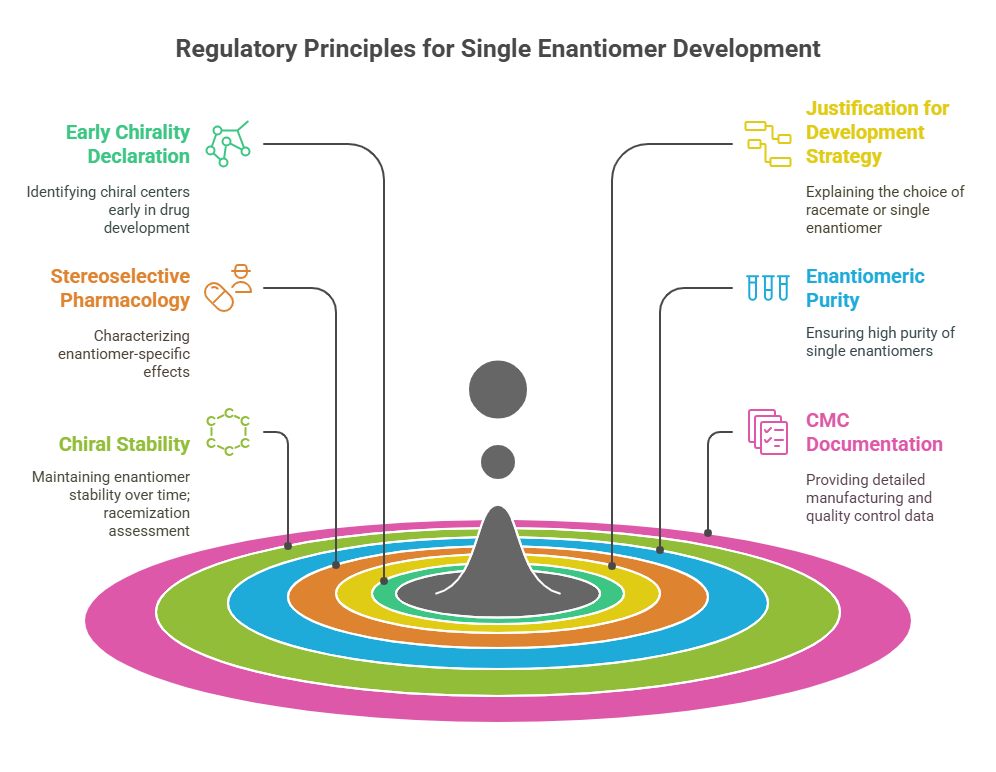
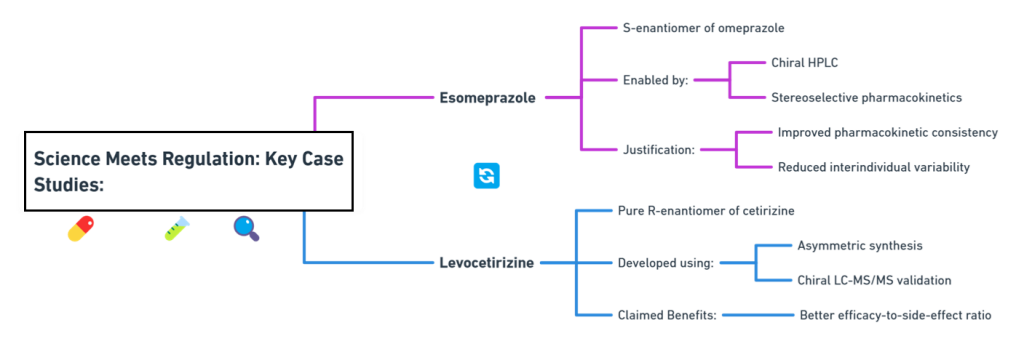
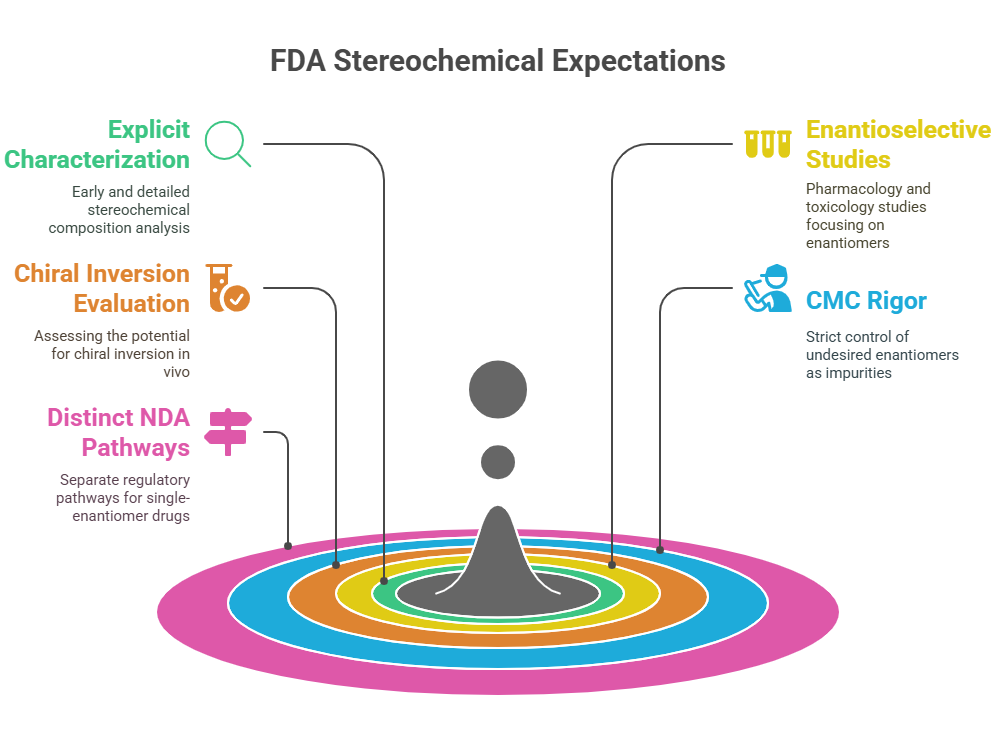
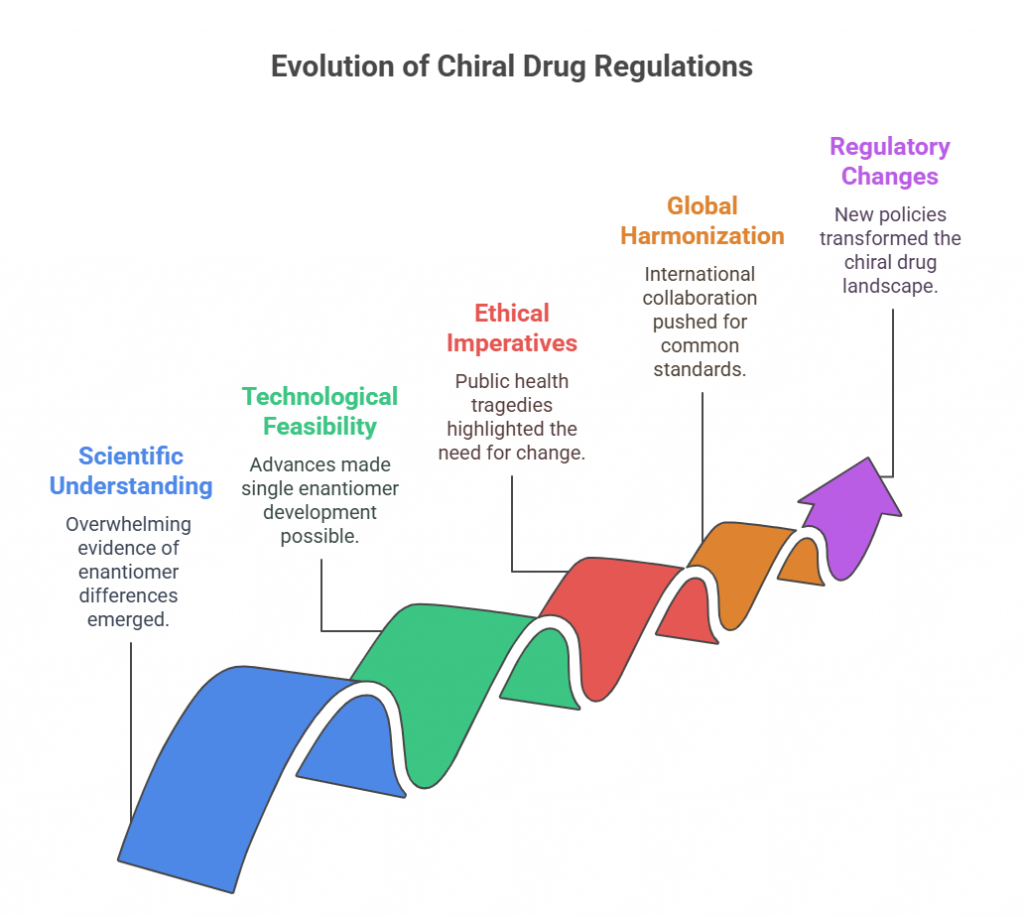
“Approval is not the end of a molecule’s journey – the mirror must be watched, studied, and questioned throughout its life.” Introduction The development and approval of chiral drugs, especially single-enantiomer entities, marked a new chapter in pharmaceutical science. However, approval is only the beginning. Ensuring that a chiral drug continues to behave safely and predictably in the real world demands vigilant post-marketing surveillance, adaptive regulatory frameworks, and constant scientific reevaluation. In this final episode, …
Episode 10: Future Reflections: How the Story of Chiral Drug Regulation Continues Read More »