The Fischer convention was introduced to specify the configuration of a stereogenic center and uses the symbols D and L. The use of capital letters is to differentiate from the “d” / “l” notation (optical descriptor) described earlier. This nomenclature system has become obsolete. In general the D/L system of nomenclature is superseded by the Cahn-Ingold-Prelog (CIP) rule to describe the configuration of a stereogenic/chiral center. But D-/L-system of naming is still employed to designate the configuration of amino acids and sugars. So it is important to understand D-/L- system of naming enantiomers.
Fischer convention
The need for consistency in stereochemical designation prompted Emil Fischer to use C-5 of the dextrorotatory, (+)-, enantiomer of glucose as a starting point. This molecule was drawn in Fischer projection formula.
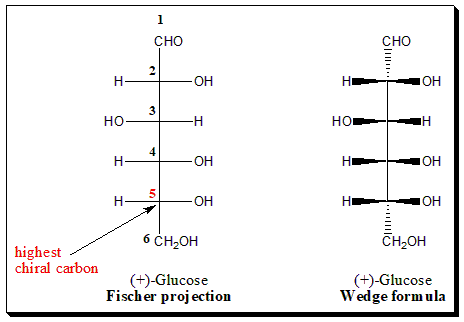
(+)-Glucose was degraded by Fischer to an aldotriose, (+)-Glyceraldehyde, in which the only stereogenic center originates from C-5 of the parent molecule. Arbitrarily, Fischer assigned the configuration (I) to (+)-Glyceraldehyde, which became D-(+)-Glyceraldehyde (OH on the right-hand side position) and the configuration (II) to (-)-Glyceraldehyde, which became L-(-)-Glyceraldehyde (OH on the left-hand side position).
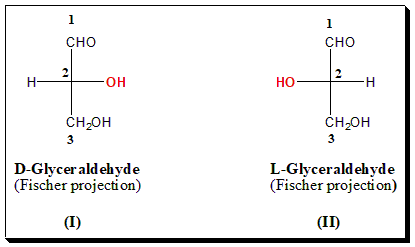
In this system, the enantiomers are named with reference to D- and L-Glyceraldehyde which is taken as the standard for comparison. The structure of the chiral molecule should be represented in the Fischer projection formula. If the hydroxyl group attached to the highest chiral carbon is on the right-hand side it is referred to as D-series and if on the left-hand side it is called L-series. The configurational assignment based on chemically relating to chiral molecules to either D- or L-Glyceraldehyde is known as the genetic nomenclature.
Case study: Glucose
Glucose is six carbon sugar (aldohexose). To apply Fischer convention focus on the placement of the -OH group attached to the highest chiral carbon (in this case C-5 (highlighted in red color).
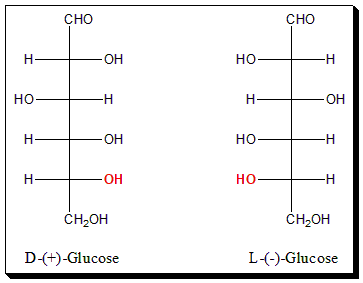
In (+)-Glucose, the -OH group attached to the highest chiral carbon (C-5) is on the right-hand side of the carbon skeleton. Hence it is assigned the D-configuration. Whereas in the case of (-)-Glucose the -OH group attached to carbon (C-5) is on the left-hand side and hence assigned L-configuration.
Amino acid Convention – α-carbon
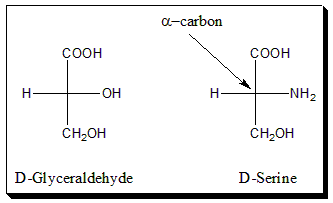
The absolute configuration at the α-carbon atom of the α-amino acids is designated by the prefixed capital letter D or L to indicate a formal relationship to D- or L-serine and thus to D- or L-Glyceraldehyde. The configuration is determined by the location of amino group (-NH2) on alpha carbon of amino acid, if it is on right-hand side it is called D- amino acid and if it is on left-hand side then it is called L-amino acid.
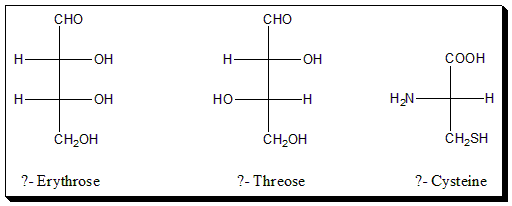
Practice exercise
Assign D-/L- configuration to the following sugars and amino acid.
References
Bernard Testa, Principles of organic stereochemistry, Marcel Dekker Inc., New York, 1979.
Hartung, Walter H.; Andrako, John (1961). “Stereochemistry”. Journal of Pharmaceutical Sciences. 50 (10): 805–818. doi:10.1002/jps.2600501002
Slocum, D. W.; Surgarman, D.; Tucker, S. P. (1971). “The two faces of D and L nomenclature”. Journal of Chemical Education. 48 (9): 597. Bibcode:1971JChEd..48..597S. DOI:10.1021/ed048p597
Chiral drugs. Wikipedia, Wikipedia Foundation, 07/03/2022. https://en.wikipedia.org/wiki/Chiral_drugs

It’s pretty good to read the concepts as well effective of gaining knowledge is too good like this blog. I really learned a lot.
I request you to add up following topics
– Asymmetric synthesis
– Racemic mixtures
With grateful~mj
Thank you!!
-karpagam
Thank you so much…This added lot of knowledge on isomers
I have studied cis and trans , E and Z notations ,meso compound , d and l configuration. It was very useful.
Very useful 👍
Easy to understand