Synopsis
Recently, an eye-opening commentary by Israel Agranat & Ilaria D’Acquarica, published in the European Journal of Pharmaceutical Sciences (2025), challenges a long-standing assumption in drug development: that single enantiomers are always the superior choice to racemic or diastereomeric mixtures in terms of efficacy and safety. Through historical analysis, case studies, and regulatory context, the authors argue that racemates should not be automatically dismissed during drug development.
The article outlines both the advantages of racemates (e.g., synergy, cost-effectiveness, broader activity) and advantages of single enantiomers (e.g., cleaner pharmacokinetics, selective targeting, personalized dosing). It cautions against the blanket belief that single enantiomers are always better and calls for a case-by-case, evidence-based approach to chiral drug development.
In a world where time is limited and attention is fleeting, even seasoned medicinal and chiral chemists may struggle to dive into dense research papers. This blog transforms complex scientific ideas into clear, visually compelling stories – making learning not only easier, but more memorable and meaningful.
Making Chirality Click – One Visual at a Time.
Core Thesis
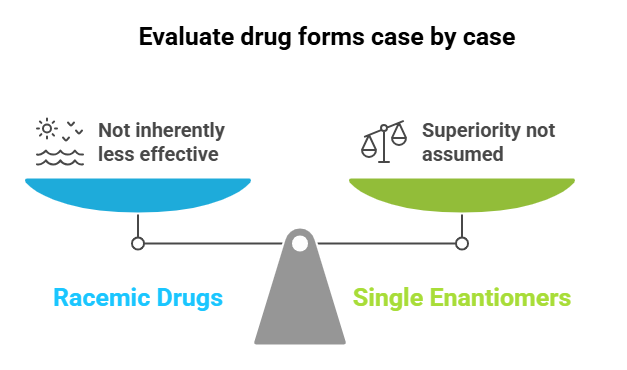
Racemic drugs (mixtures of enantiomers) are not inherently less effective or less safe than their single-enantiomer counterparts. The superiority of single enantiomers should not be assumed; both options must be evaluated case by case.
Historical Context & Regulatory Influence
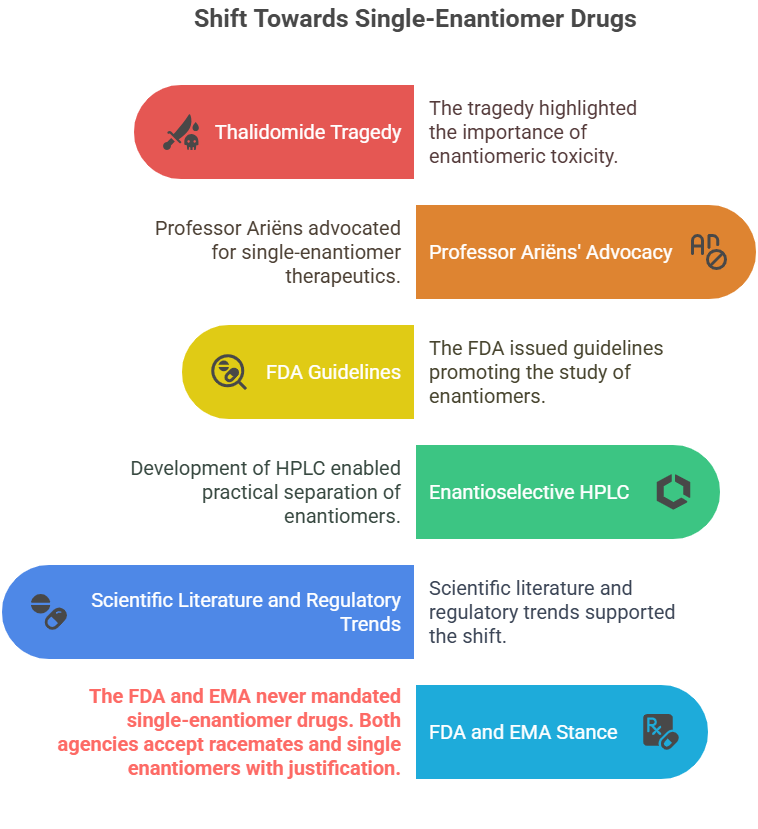
However, the FDA and EMA never mandated single-enantiomer drugs – both racemates and single enantiomers are acceptable with justification.
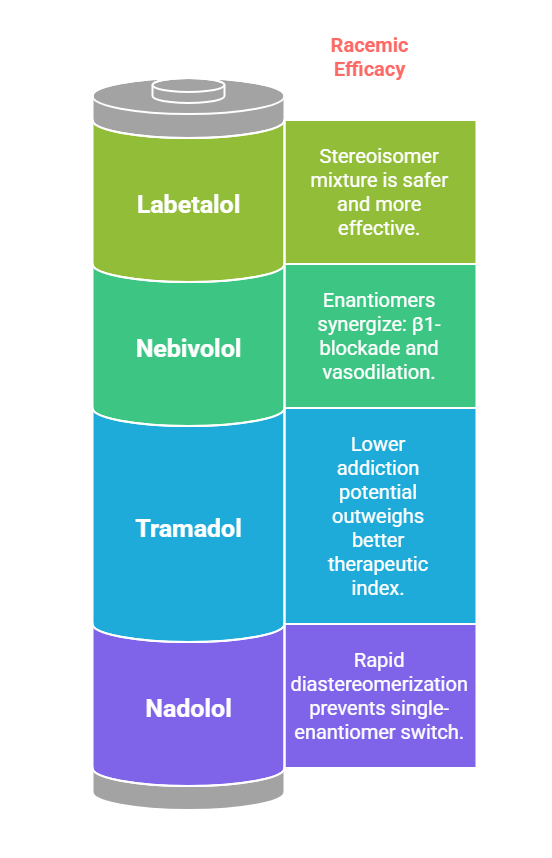
Case Studies Favoring Racemates
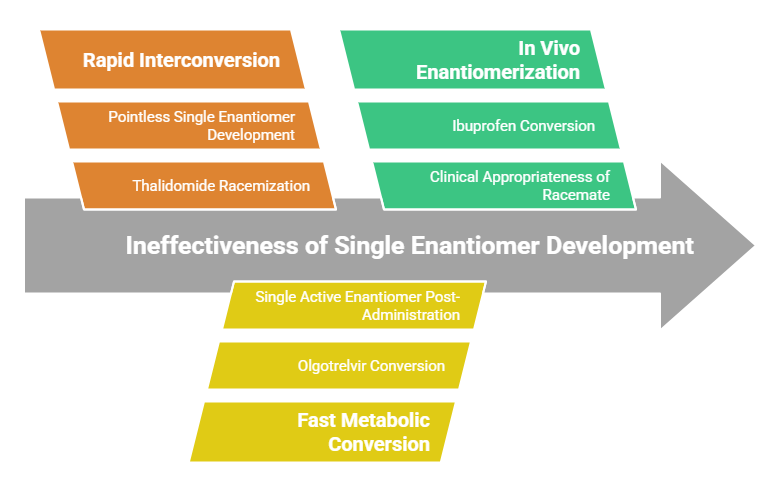
Challenges in Developing Single Enantiomers
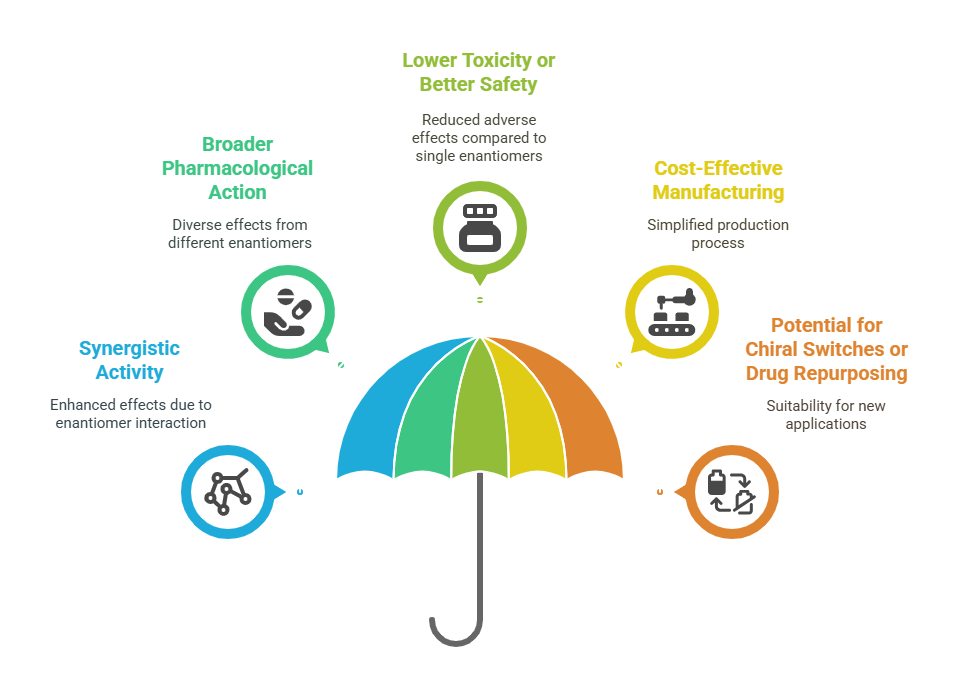
Benefits of Racemic drugs
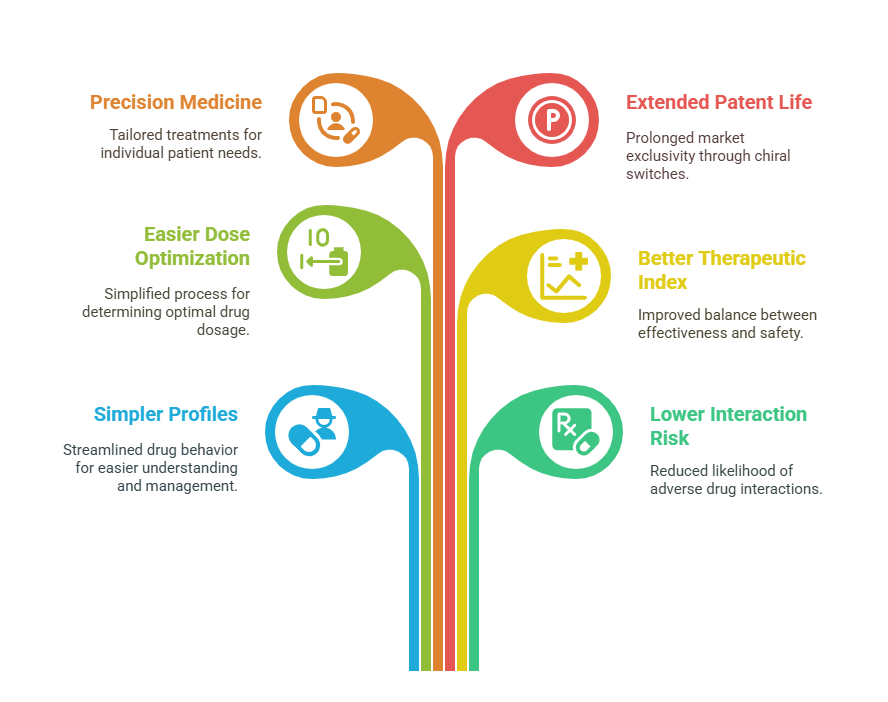
Unveiling Benefits of Single-Enantiomer Drugs
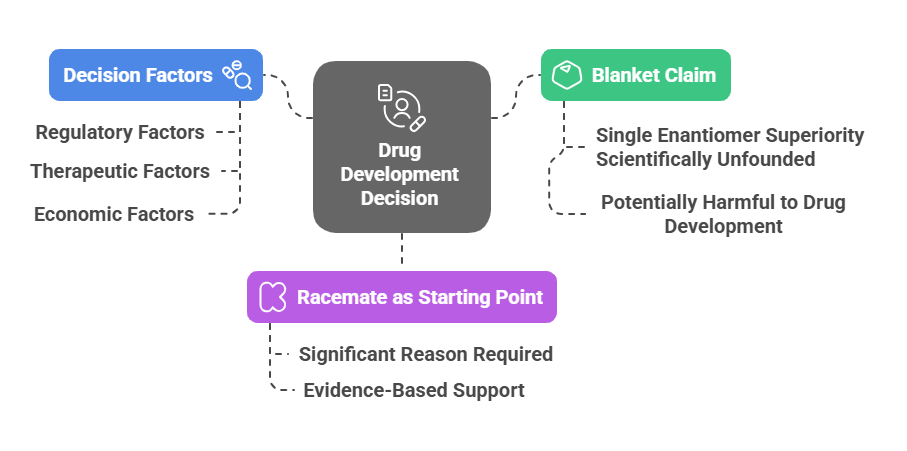
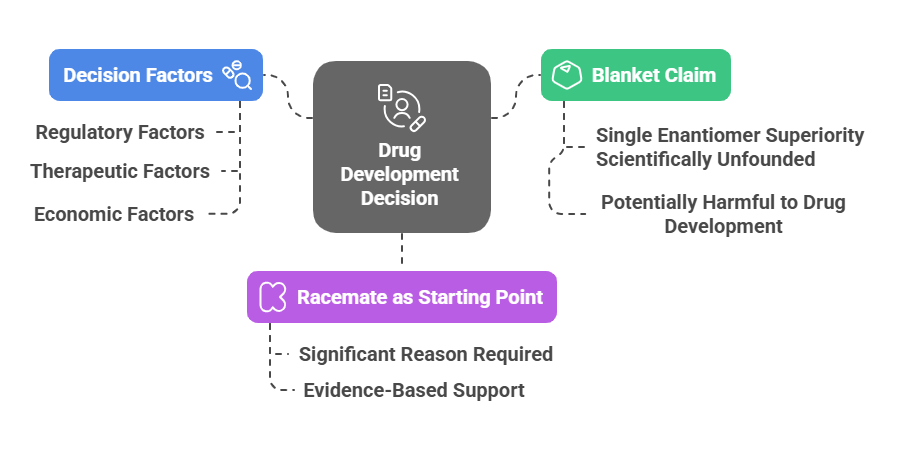
Conclusion: Decision making in chiral drug development
The authors propose a pragmatic strategy: start with the racemate unless compelling pharmacological or safety data justify a switch to a single enantiomer.
References
Israel Agranat & Ilaria D’Acquarica (2025), Racemic drugs are not necessarily less efficacious and less safe than their single-enantiomer components. European Journal of Pharmaceutical Sciences. https://doi.org/10.1016/j.ejps.2025.107082 and references therein.