Enantiomers are pair of molecules that exist in two forms that are mirror images of one another but cannot be superimposed one upon the other. They are also referred to by chemists as chiral twins or handed molecules. Each twin is called an enantiomer. Naming of enantiomers is important to understand ‘which structure refers to which enantiomer?’.
The chirality of organic molecules is described by the Cahn-Ingold-Prelog (CIP) system. This system is also referred to as the R/S convention or Sequence rule. This convention forms a part of the IUPAC rules of nomenclature. According to this system enantiomers are classified as ‘R’ (right from the Latin word ‘rectus’) and ‘S’ (left from the Latin word ‘Sinister’) absolute configuration based on the spatial orientation around the chiral center.. The most commonly encountered stereogenic unit that confers chirality to drug molecules is chiral center (stereogenic center). Stereogenic center can be due to the presence of tetrahedral tetra coordinate atoms (C,N,P) and pyramidal tricoordinate atoms (N,S).
The procedure is fairly straightforward for simple compounds. First you assign priorities to the groups attached around the chiral center. Next, you rotate the molecule so that the lowest priority group is pointing towards the back (away from you, the observer). Finally, you examine the remaining group priorities and determine if they are now arranged so that the priority decreases clockwise (R, for rectus) or counterclockwise (S, for sinister).
These rules are restated below, with example. The natural alanine goes into the making of proteins and is taken for illustrative purpose. It is a simple acyclic organic molecule with a chiral center.
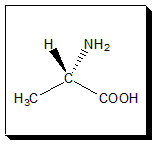
1. Identifying the chiral center
Look for the atom in the molecule with four different ligands or substituents or atoms.
2. Assigning priorities for the substituents
Examine the four atoms directly attached to the chiral center in question. Assign priorities according to the sequence rule i.e., in order of decreasing atomic number; the atom with the highest atomic number is #1, the next is #2, etc.
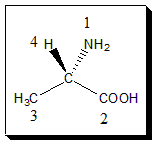
Alanine’s chiral center carries one N atom (atomic number 7), two C atoms (atomic number 6), and one H atom (atomic number 1). So, we assign priority 1 to the NH2 group, because N has the highest atomic number. Priorities 2 and 3 will be assigned to the CO2H and the CH3 groups, and priority 4 to the hydrogen atom; but we need a way of deciding which of CO2H and CH3 takes priority over the other. If two (or more) of the atoms attached to the chiral center are identical, then we assign priorities to these two by assessing the atoms attached to those atoms. In this case, one of the carbon atoms carries oxygen atoms (atomic number 8), and one carries only hydrogen atoms (atomic number 1). So CO2H is higher priority that CH3; in other words, CO2H gets priority 2 and CH3 priority 3.
Note: If a decision regarding priority cannot be reached using atomic number of the first atom, compare the atomic numbers of the second atoms in each substituent, then the third, etc., until a difference is found; Multiple bonds count twice (or three times) when examining substituents, as the case may be.
3. Viewing the molecule
. Once the priorities have been assigned, rotate the molecule in space so that the lowest priority group is pointing back. In our example, alanine, H is priority 4, so we need to look at the molecule with the H atom pointing away from you, like this.
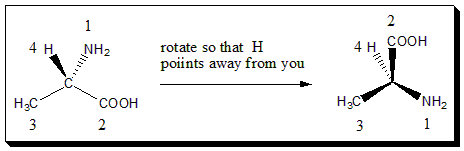
4. The wheel or eye rotation
Connect the three remaining groups in order of decreasing priority and examine the direction of the resulting rotation. Mentally move from substituent priority 1 to 2 to 3. If you r eyes move in a clockwise manner, assign the label R (rectus; right) to the chiral center; if your eyes traces a counter-clockwise manner, assign the label S (sinister; left) to the chiral center.
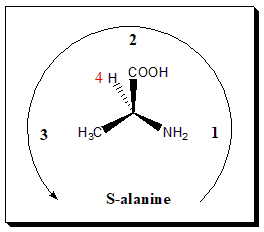
An easy way of visualizing this is to imagine turning a steering wheel in the direction of the numbering. If you are turning your wheel to the right, you assign R; if you are turning to the left you assign S. For our molecule of natural alanine, if we move from NH2 (1) to CO2H (2) toCH3 (3) we’re going anticlockwise (turning to the left), so we label this enantiomer (S)-alanine.
Let us take ibuprofen, the non-steroidal anti-inflammatory drug, as a case study. This molecule has one stereogenic carbon atom in the structure. Hence the molecule exists as an enantiomeric pair.
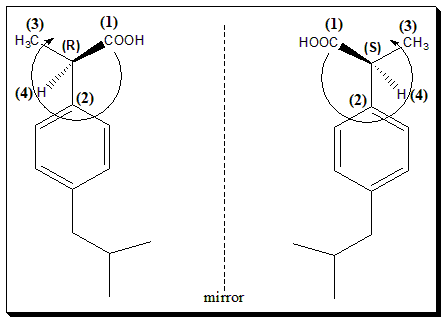
To assign absolute configuration to the chiral molecule let us apply CIP rule. The four ligand attached to the chiral carbon are –COOH, C6H5-, -CH3, and –H atom. According to sequence rule –COOH is assigned (1), C6H5– assigned (2), -CH3 (3), and –H atom (4). Then the molecule is viewed keeping the lowest priority group (here –H atom) away from the observer. When the eye traces a clockwise rotation it is labeled R- configuration (R-Ibuprofen) and if eye race counterclockwise it is labeled S-configuration (S-Ibuprofen). Note that enantiomers will have opposite configuration at all the stereogenic centers.
Practice exercises
Name the following chiral molecules using R/S system
Lactic acid:
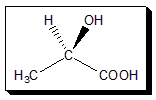
Lactic acid is an acyclic organic acid. Lactic acid is produced by bacterial action on milk; it’s also produced in your muscles when they have to work with an insufficient supply of oxygen, such as during bursts of vigorous exercise. Lactic acid produced by fermentation is often racemic, though certain species of bacteria produce solely (R)-lactic acid. On the other hand, lactic acid produced by anaerobic respiration in muscles has the (S)- configuration.
Thalidomide:
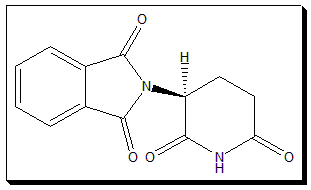
Thalidomide, a glutamic acid derivative, is a chiral drug, The molecule is a cyclic structure with a stereogenic carbon in the glutarimide ring. Hence it exists as an equimolar mixture of S(-)- and R(+)-enantiomers. It was marketed as racemic mixture.
Penicillamine:
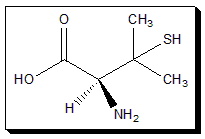
Penicillamine is a chiral drug with one stereogenic carbon and exists as a pair of enantiomers. The (S)-enantiomer, has the beneficial antiarthritic property while its mirror-image version, (R)-penicillamine, is extremely toxic.
References
Cahn, B. S.; lngold, C. K; Prelog, V (1956). “The specification of asymmetric configuration in organic chemistry”. Experientia. 12 (3): 81–88. doi:10.1007/BF02157171. S2CID 43026989
Mislow, Kurt; Siegel, Jay (1984). “Stereoisomerism and local chirality”. Journal of the American Chemical Society. 106 (11): 3319–3328. doi:10.1021/ja00323a043
Chiral drugs. Wikipedia, Wikipedia Foundation, 02/03/2022. https://en.wikipedia.org/wiki/Chiral_drugs
https://crab.rutgers.edu/~alroche/Ch05.pdf
Bernard Testa, Principles of organic stereochemistry, Marcel Dekker Inc.,, New York, 1979.

Great post I would like to thank you for the efforts you have made in writing this interesting and knowledgeable article.